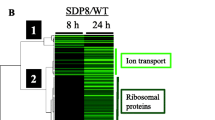
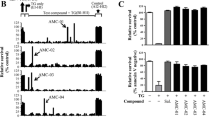
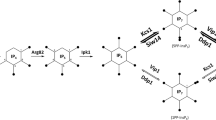
Abstract
Chlorpromazine (CPZ) is a small permeable cationic amphiphilic molecule that inserts into membrane bilayers and binds to anionic lipids such as poly-phosphoinositides (PIs). Since PIs play important roles in many cellular processes, including signaling and membrane trafficking pathways, it has been proposed that CPZ affects cellular growth functions by preventing the recruitment of proteins with specific PI-binding domains. In this study, we have investigated the biological effects of CPZ in the yeast Saccharomyces cerevisiae. We screened a collection of approximately 4,800 gene knockout mutants, and found that mutants defective in membrane trafficking between the late-Golgi and endosomal compartments are highly sensitive to CPZ. Microscopy and transport analyses revealed that CPZ affects membrane structure of organelles, blocks membrane transport and activates the unfolded protein response (UPR). In addition, CPZ-treatment induces phosphorylation of the translation initiation factor (eIF2α), which reduces the general rate of protein synthesis and stimulates the production of Gcn4p, a major transcription factor that is activated in response to environmental stresses. Altogether, our results reveal that membrane stress within the cells rapidly activates an important gene expression program, which is followed by a general inhibition of protein synthesis. Remarkably, the increase of phosphorylated eIF2α and protein synthesis inhibition were also detected in CPZ-treated NIH-3T3 fibroblasts, suggesting the existence of a conserved mechanism of translational regulation that operates during a membrane stress.








Similar content being viewed by others
Abbreviations
- CPZ :
-
Chlorpromazine
- Tm :
-
Tunicamycin
- 3-AT :
-
3-Aminotriazole
- UPR :
-
Unfolded protein response
- ER :
-
Endoplasmic reticulum
- PI :
-
Phosphoinositide
- PS :
-
Phosphatidylserine
- PA :
-
Phosphatidylamine
- PE :
-
Phosphatidylethanolamine
References
Ahyayauch H, Goni FM, Bennouna M (2003) pH-dependent effects of chlorpromazine on liposomes and erythrocyte membranes. J Liposome Res 13:147–155
Bankaitis VA, Aitken JR, Cleves AE, Dowhan W (1990) An essential role for a phospholipid transfer protein in yeast Golgi function. Nature 347:561–562
Beck T, Hall MN (1999) The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature 402:689–692
Cameroni E, De Virgilio C, Deloche O (2006) Phosphatidylinositol 4-phosphate is required for translation initiation in Saccharomyces cerevisiae. J Biol Chem 281:38139–38149
Capuano B, Crosby IT, Lloyd EJ (2002) Schizophrenia: genesis, receptorology and current therapeutics. Curr Med Chem 9:521–548
Chang HJ, Jesch SA, Gaspar ML, Henry SA (2004) Role of the unfolded protein response pathway in secretory stress and regulation of INO1 expression in Saccharomyces cerevisiae. Genetics 168:1899–1913
Chen EJ, Kaiser CA (2003) LST8 negatively regulates amino acid biosynthesis as a component of the TOR pathway. J Cell Biol 161:333–347
Chen JY, Brunauer LS, Chu FC, Helsel CM, Gedde MM, Huestis WH (2003) Selective amphipathic nature of chlorpromazine binding to plasma membrane bilayers. Biochim Biophys Acta 1616:95–105
Cherkasova VA, Hinnebusch AG (2003) Translational control by TOR and TAP42 through dephosphorylation of eIF2alpha kinase GCN2. Genes Dev 17:859–872
Chuang JS, Schekman RW (1996) Differential trafficking and timed localization of two chitin synthase proteins, Chs2p and Chs3p. J Cell Biol 135:597–610
Cox JS, Shamu CE, Walter P (1993) Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 73:1197–1206
de la Cruz J, Iost I, Kressler D, Linder P (1997) The p20 and Ded1 proteins have antagonistic roles in eIF4E-dependent translation in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 94:5201–5206
De Virgilio C, Burckert N, Bell W, Jeno P, Boller T, Wiemken A (1993) Disruption of TPS2, the gene encoding the 100-kDa subunit of the trehalose-6-phosphate synthase/phosphatase complex in Saccharomyces cerevisiae, causes accumulation of trehalose-6-phosphate and loss of trehalose-6-phosphate phosphatase activity. Eur J Biochem 212:315–323
Deloche O, de la Cruz J, Kressler D, Doere M, Linder P (2004) A membrane transport defect leads to a rapid attenuation of translation initiation in Saccharomyces cerevisiae. Mol Cell 13:357–366
Dever TE (1999) Translation initiation: adept at adapting. Trends Biochem Sci 24:398–403
Dever TE, Feng L, Wek RC, Cigan AM, Donahue TF, Hinnebusch AG (1992) Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell 68:585–596
Drysdale CM, Duenas E, Jackson BM, Reusser U, Braus GH, Hinnebusch AG (1995) The transcriptional activator GCN4 contains multiple activation domains that are critically dependent on hydrophobic amino acids. Mol Cell Biol 15:1220–1233
Dubouloz F, Deloche O, Wanke V, Cameroni E, De Virgilio C (2005) The TOR and EGO protein complexes orchestrate microautophagy in yeast. Mol Cell 19:15–26
Efe JA, Plattner F, Hulo N, Kressler D, Emr SD, Deloche O (2005) Yeast Mon2p is a highly conserved protein that functions in the cytoplasm-to-vacuole transport pathway and is required for Golgi homeostasis. J Cell Sci 118:4751–4764
Foti M, Audhya A, Emr SD (2001) Sac1 lipid phosphatase and Stt4 phosphatidylinositol 4-kinase regulate a pool of phosphatidylinositol 4-phosphate that functions in the control of the actin cytoskeleton and vacuole morphology. Mol Biol Cell 12:2396–2411
Franzusoff A, Lauze E, Howell KE (1992) Immuno-isolation of Sec7p-coated transport vesicles from the yeast secretory pathway. Nature 355:173–175
Frey S, Pool M, Seedorf M (2001) Scp160p, an RNA-binding, polysome-associated protein, localizes to the endoplasmic reticulum of Saccharomyces cerevisiae in a microtubule-dependent manner. J Biol Chem 276:15905–159012
Frolich KW, Aarbakke GM, Holmsen H (1992) Chlorpromazine increases the turnover of metabolically active phosphoinositides and elevates the steady-state level of phosphatidylinositol-4-phosphate in human platelets. Biochem Pharmacol 44:2013–2020
Fuller RS, Sterne RE, Thorner J (1988) Enzymes required for yeast prohormone processing. Annu Rev Physiol 50:345–362
Gaynor EC, Emr SD (1997) COPI-independent anterograde transport: cargo-selective ER to Golgi protein transport in yeast COPI mutants. J Cell Biol 136:789–802
Guthrie C, Fink GR (1991) Guide to yeast genetics and molecular biology. Methods Enzymol 194:3–21
Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D (2000) Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5:897–904
Hinnebusch AG (2005) Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol 59:407–450
Holcik M, Sonenberg N (2005) Translational control in stress and apoptosis. Nat Rev Mol Cell Biol 6:318–327
Huffaker TC, Hoyt MA, Botstein D (1987) Genetic analysis of the yeast cytoskeleton. Annu Rev Genet 21:259–284
Julius D, Schekman R, Thorner J (1984) Glycosylation and processing of prepro-alpha-factor through the yeast secretory pathway. Cell 36:309–318
Jutila A, Soderlund T, Pakkanen AL, Huttunen M, Kinnunen PK (2001) Comparison of the effects of clozapine, chlorpromazine, and haloperidol on membrane lateral heterogeneity. Chem Phys Lipids 112:151–163
Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290:1717–1721
Ktistakis NT, Brown HA, Waters MG, Sternweis PC, Roth MG (1996) Evidence that phospholipase D mediates ADP ribosylation factor-dependent formation of Golgi coated vesicles. J Cell Biol 134:295–306
Kushnirov VV (2000) Rapid and reliable protein extraction from yeast. Yeast 16:857–860
Leber JH, Bernales S, Walter P (2004) IRE1-independent gain control of the unfolded protein response. PLoS Biol 2:E235
Mendelsohn BA, Li AM, Vargas CA, Riehman K, Watson A, Fridovich-Keil JL (2003) Genetic and biochemical interactions between SCP160 and EAP1 in yeast. Nucleic Acids Res 31:5838–5847
Mizuta K, Warner JR (1994) Continued functioning of the secretory pathway is essential for ribosome synthesis. Mol Cell Biol 14:2493–2502
Natarajan K, Meyer MR, Jackson BM, Slade D, Roberts C, Hinnebusch AG, Marton MJ (2001) Transcriptional profiling shows that Gcn4p is a master regulator of gene expression during amino acid starvation in yeast. Mol Cell Biol 21:4347–4368
Nierras CR, Warner JR (1999) Protein kinase C enables the regulatory circuit that connects membrane synthesis to ribosome synthesis in Saccharomyces cerevisiae. J Biol Chem 274:13235–13241
Novick P, Field C, Schekman R (1980) Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 21:205–215
Palmer LK, Wolfe D, Keeley JL, Keil RL (2002) Volatile anesthetics affect nutrient availability in yeast. Genetics 161:563–574
Patil CK, Li H, Walter P (2004) Gcn4p and novel upstream activating sequences regulate targets of the unfolded protein response. PLoS Biol 2:E246
Peters C, Andrews PD, Stark MJ, Cesaro-Tadic S, Glatz A, Podtelejnikov A, Mann M, Mayer A (1999) Control of the terminal step of intracellular membrane fusion by protein phosphatase 1. Science 285:1084–1087
Raucher D, Sheetz MP (2001) Phospholipase C activation by anesthetics decreases membrane–cytoskeleton adhesion. J Cell Sci 114:3759–3766
Raught B, Peiretti F, Gingras AC, Livingstone M, Shahbazian D, Mayeur GL, Polakiewicz RD, Sonenberg N, Hershey JW (2004) Phosphorylation of eucaryotic translation initiation factor 4B Ser422 is modulated by S6 kinases. Embo J 23:1761–1769
Rieder SE, Banta LM, Kohrer K, McCaffery JM, Emr SD (1996) Multilamellar endosome-like compartment accumulates in the yeast vps28 vacuolar protein sorting mutant. Mol Biol Cell 7:985–999
Robinson JS, Klionsky DJ, Banta LM, Emr SD (1988) Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol Cell Biol 8:4936–4948
Rose MD, Winston F, Hieter P (1988) Laboratory course manual for method in yeast genetics. Cold Spring Habor Laboratory, New York
Rossanese OW, Reinke CA, Bevis BJ, Hammond AT, Sears IB, O’Connor J, Glick BS (2001) A role for actin, Cdc1p, and Myo2p in the inheritance of late Golgi elements in Saccharomyces cerevisiae. J Cell Biol 153:47–62
Rothman JE, Wieland FT (1996) Protein sorting by transport vesicles. Science 272:227–234
Russo P, Kalkkinen N, Sareneva H, Paakkola J, Makarow M (1992) A heat shock gene from Saccharomyces cerevisiae encoding a secretory glycoprotein. Proc Natl Acad Sci USA 89:3671–3675
Schorr M, Then A, Tahirovic S, Hug N, Mayinger P (2001) The phosphoinositide phosphatase Sac1p controls trafficking of the yeast Chs3p chitin synthase. Curr Biol 11:1421–1426
Seeger M, Payne GS (1992) Selective and immediate effects of clathrin heavy chain mutations on Golgi membrane protein retention in Saccharomyces cerevisiae. J Cell Biol 118:531–540
Sheetz MP, Singer SJ (1974) Biological membranes as bilayer couples. A molecular mechanism of drug–erythrocyte interactions. Proc Natl Acad Sci USA 71:4457–4461
Steffensen L, Pedersen PA (2006) Heterologous expression of membrane and soluble proteins derepresses GCN4 mRNA translation in the yeast Saccharomyces cerevisiae. Eukaryot Cell 5:248–261
Stevens TH, Rothman JH, Payne GS, Schekman R (1986) Gene dosage-dependent secretion of yeast vacuolar carboxypeptidase Y. J Cell Biol 102:1551–1557
Trichard C, Paillere-Martinot ML, Attar-Levy D, Recassens C, Monnet F, Martinot JL (1998) Binding of antipsychotic drugs to cortical 5-HT2A receptors: a PET study of chlorpromazine, clozapine, and amisulpride in schizophrenic patients. Am J Psychiatr 155:505–508
Wang LH, Rothberg KG, Anderson RG (1993) Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol 123:1107–1117
Wek RC, Cannon JF, Dever TE, Hinnebusch AG (1992) Truncated protein phosphatase GLC7 restores translational activation of GCN4 expression in yeast mutants defective for the eIF-2 alpha kinase GCN2. Mol Cell Biol 12:5700–5710
Zhou M, Schekman R (1999) The engagement of Sec61p in the ER dislocation process. Mol Cell 4:925–34
Acknowledgments
We are extremely grateful for continuous support from Costa Georgopoulos. We thank S. Emr, T. R. Graham, R. Schekman, A. Hinnebusch and T. Dever for providing strains, plasmids and antibodies. We also thank D. Ang for a critical reading of the manuscript and the PFMU at the Geneva Medical Faculty for access to electron microscope and ancillary equipments. This work was supported by grants from the Swiss National Science Foundation and the Canton of Geneva to C. Georgopoulos (FN-31-654039), M.F. (FN-3100A0-104489), PL (FN-3100A0-105894/1) and CDV (PP00A-106754/1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Gerhard Braus.
Rights and permissions
About this article
Cite this article
De Filippi, L., Fournier, M., Cameroni, E. et al. Membrane stress is coupled to a rapid translational control of gene expression in chlorpromazine-treated cells. Curr Genet 52, 171–185 (2007). https://doi.org/10.1007/s00294-007-0151-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-007-0151-0