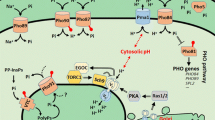
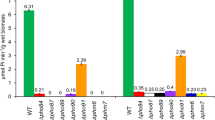
Abstract
Membrane transport systems active in cellular inorganic phosphate (Pi) acquisition play a key role in maintaining cellular Pi homeostasis, independent of whether the cell is a unicellular microorganism or is contained in the tissue of a higher eukaryotic organism. Since unicellular eukaryotes such as yeast interact directly with the nutritious environment, regulation of Pi transport is maintained solely by transduction of nutrient signals across the plasma membrane. The individual yeast cell thus recognizes nutrients that can act as both signals and sustenance. The present review provides an overview of Pi acquisition via the plasma membrane Pi transporters of Saccharomyces cerevisiae and the regulation of internal Pi stores under the prevailing Pi status.




Similar content being viewed by others
References
André B (1995) An overview of membrane transport proteins in Saccharomyces cerevisiae. Yeast 11:1575–1611
Andreeva NA, Kulakovskaya TV, Kulaev IS (2001) Two exopolyphosphatases of the cytosol of the yeast S. cerevisiae: comparative characteristics. Biochemistry (Mosc) 66:147–153
Andrews B, Measday V (1998) The cyclin family of budding yeast: abundant use of a good idea. Trends Genet 14:66–72
Arima K, Oshima T, Kubota I, Nakamura N, Mizunaga T, Toh-e A (1983) The nucleotide sequence of the yeast PHO5 gene: a putative precursor of repressible acid phosphatase contains a signal peptide. Nucleic Acids Res 11:1657–1672
Arndt KT, Styles C, Fink GR (1987) Multiple global regulators control HIS4 transcription in yeast. Science 237:874–880
Balch WE (1990) Small GTP-binding proteins in vesicular transport. Trends Biochem Sci 15:473–477
Barbaric S, Münsterkötter M, Svaren J, Horz W (1996) The homeodomain protein Pho2 and the basic-helix-loop-helix protein Pho4 bind DNA cooperatively at the yeast PHO5 promoter. Nucleic Acids Res 24:4479–4486
Barbaric S, Münsterkötter M, Goding C, Horz W (1998) Cooperative Pho2–Pho4 interactions at the PHO5 promoter are critical for binding of Pho4 to UASp1 and for efficient transactivation by Pho4 at UASp2. Mol Cell Biol 18:2629–2639
Beauvoit B, Rigoulet M, Guerin B, Canioni P (1989) Polyphosphates as a source of high energy phosphates in yeast mitochondria: a 31P-NMR study. FEBS Lett 252:17–21
Beauvoit B, Rigoulet M, Raffard G, Canioni P, Guerin B (1991) Differential sensitivity of the cellular compartments of Saccharomyces cerevisiae to protonophoric uncoupler under fermentative and respiratory energy supply. Biochemistry 30:11212–11220
Berhe A, Fristedt U, Persson BL (1995) Expression and purification of the high-affinity phosphate transporter of Saccharomyces cerevisiae. Eur J Biochem 227:566–572
Berhe A, Zvyagilskaya R, Lagerstedt JO, Pratt JR, Persson BL (2001) Properties of the cysteine-less Pho84 phosphate transporter of Saccharomyces cerevisiae. Biochem Biophys Res Commun 287:837–842
Bhoite LT, Allen JM, Garcia E, Thomas LR, Gregory ID, Voth WP, Whelihan K, Rolfes RJ, Stillman DJ (2002) Mutations in the Pho2 (Bas2) transcription factor that differentially affect activation with its partner proteins Bas1, Pho4, and Swi5. J Biol Chem 277:37612–37618
Bisson LF, Coons DM, Kruckeberg AL, Lewis DA (1993) Yeast sugar transporters. Crit Rev Biochem Mol Biol 28:259–308
Blasco F, Ducet G, Azoulay E (1976) Demonstration of 2 phosphate transport systems in Candida tropicalis. Biochimie 58:351–357
Boles E, Hollenberg CP (1997) The molecular genetics of hexose transport in yeasts. FEMS Microbiol Rev 21:85–111
Booth JW, Guidotti G (1997) Phosphate transport in yeast vacuoles. J Biol Chem 272:20408–20413
Bork P (1993) Hundreds of ankyrin-like repeats in functionally diverse proteins: mobile modules that cross phyla horizontally? Proteins 17:363–374
Borst-Pauwels GWFH (1981) Ion transport in yeast. Biochim Biophys Acta 650:88–127
Borst-Pauwels GWFH (1993) Mutual interaction of ion uptake and membrane potential. Biochim Biophys Acta 1145:15–24
Borst-Pauwels GW, Peters PH (1977) Effect of the medium pH and the cell pH upon the kinetical parameters of phosphate uptake by yeast. Biochim Biophys Acta 466:488–495
Borst-Pauwels GWFH, Peters PHJ (1987) Phosphate uptake in Saccharomyces cerevisiae. In: Torriani-Gorini A, Rothman FG, Silver S, Wright A, Yagil E (eds) Phosphate metabolism and cellular regulation of microorganisms. ASM Press, Washington, D.C., pp 205–209
Bostian KA, Lemire JM, Cannon LE, Halvorson HO (1980) In vitro synthesis of repressible yeast acid phosphatase: identification of multiple mRNAs and products. Proc Natl Acad Sci USA 77:4504–4508
Bostian KA, Lemire JM, Halvorson HO (1983) Physiological control of repressible acid phosphatase gene transcripts in Saccharomyces cerevisiae. Mol Cell Biol 3:839–853
Böttger P, Pedersen L (2002) Two highly conserved glutamate residues critical for type III sodium-dependent phosphate transport revealed by uncoupling transport function from retroviral receptor function. J Biol Chem 277:42741–42747
Bourne RM (1990) A 31P-NMR study of phosphate transport and compartmentation in Candida utilis. Biochim Biophys Acta 1055:1–9
Bourne RM (1991) Net phosphate transport in phosphate-starved Candida utilis: relationships with pH and K+. Biochim Biophys Acta 1067:81–88
Braus G, Mösch HU, Vogel K, Hinnen A, Hütter R (1989) Interpathway regulation of the TRP4 gene of yeast. EMBO J 8:939–945
Brazas RM, Stillman DJ (1993) The Swi5 zinc-finger and Grf10 homeodomain proteins bind DNA cooperatively at the yeast HO promoter. Proc Natl Acad Sci USA 90:11237–11241
Brazas RM, Bhoite LT, Murphy MD, Yu YX, Chen YY, Neklason DW, Stillman DJ (1995) Determining the requirements for cooperative DNA binding by Swi5p and Pho2p (Grf10p/Bas2p) at the HO promoter. J Biol Chem 270:29151–29161
Brown AM, Birnbaumer L (1990) Ionic channels and their regulation by G protein subunits. Annu Rev Physiol 52:197–213
Bun-ya M, Nishimura M, Harashima S, Oshima Y (1991) The PHO84 gene of Saccharomyces cerevisiae encodes an inorganic phosphate transporter. Mol Cell Biol 11:3229–3238
Bun-ya M, Harashima S, Oshima Y (1992) Putative GTP-binding protein, Gtr1, associated with the function of the Pho84 inorganic phosphate transporter in Saccharomyces cerevisiae. Mol Cell Biol 12:2958–2966
Bun-ya M, Shikata K, Nakade S, Yompakdee C, Harashima S, Oshima Y (1996) Two new genes, PHO86 and PHO87, involved in inorganic phosphate uptake in Saccharomyces cerevisiae. Curr Genet 29:344–351
Castro CD, Meehan AJ, Koretsky AP, Domach MM (1995) In situ 31P nuclear magnetic resonance for observation of polyphosphate and catabolite responses of chemostat-cultivated Saccharomyces cerevisiae after alkalinization. Appl Environ Microbiol 61:4448–4453
Causton HC, Ren B, Koh SS, Harbison CT, Kanin E, Jennings EG, et al (2001) Remodeling of yeast genome expression in response to environmental changes. Mol Biol Cell 12:333–337
Cave JW, Kremer W, Wemmer DE (2000) Backbone dynamics of sequence specific recognition and binding by the yeast Pho4 bHLH domain probed by NMR. Protein Sci 9:2354–2365
Claros MG, Heijne G von (1994) TopPredII: an improved software for membrane protein structure prediction. Comput Appl Biosci 10:685–686
Cockburn M, Earnshaw P, Eddy AA (1975) The stoichiometry of the absorption of protons with phosphate and l-glutamate by yeasts of the genus Saccharomyces. Biochem J 146:705–712
Cohen A, Perzov N, Nelson H, Nelson N (1999) A novel family of yeast chaperons involved in the distribution of V-ATPase and other membrane proteins. J Biol Chem 274:26885–26893
Costanzo MC, Crawford ME, Hirschman JE, Kranz JE, Olsen P, Robertson LS, Skrzypek MS, Braun BR, Hopkins KL, Kondu P, Lengieza C, Lew-Smith JE, Tillberg M, Garrels JI (2001) YPD, PombePD and WormPD: model organism volumes of the BioKnowledge library, an integrated resource for protein information. Nucleic Acids Res 29:75–79
Crooke E, Akiyama M, Rao NN, Kornberg A (1994) Genetically altered levels of inorganic polyphosphate in Escherichia coli. J Biol Chem 269:6290–6295
Daignan-Fornier B, Fink GR (1992) Coregulation of purine and histidine biosynthesis by the transcriptional activators BAS1 and BAS2. Proc Natl Acad Sci USA 89:6746–6750
Dawes EA, Senior PJ (1973) The role and regulation of energy reserve polymers in microorganisms. Adv Microb Physiol 10:135–266
Devenish RJ, Prescott M, Roucou X, Nagley P (2000) Insights into ATP synthase assembly and function through the molecular genetic manipulation of subunits of the yeast mitochondrial enzyme complex. Biochim Biophys Acta 1458:428–442
Dunn T, Gable K, Beeler T (1994) Regulation of cellular Ca2+ by yeast vacuoles. J Biol Chem 269:7273–7278
Espinoza FH, Ogas J, Herskowitz I, Morgan DO (1994) Cell cycle control by a complex of the cyclin HCS26 (PCL1) and the kinase PHO85. Science 266:1388–1391
Felter S, Stahl AJ (1973) Enzymes for metabolism of polyphosphates in yeast. 3. Purification and properties of polyphosphate-ADP-phosphotransferase. Biochimie 55:245–251
Ferré-D'Amaré AR, Prendergast GC, Ziff EB, Burley SK (1993) Recognition by Max of its cognate DANN trough a dimeric b/HLH/Z domain. Nature 363:38–45
Ferré-D'Amaré AR, Pognonec P, Roeder RG, Burley SK (1994) Structure and function of the b/HLH/Z domain of USF. EMBO J 13:180–189
Forsberg H, Ljungdahl PO (2001) Sensors of extracellular nutrients in Saccharomyces cerevisiae. Curr Genet 40:91–109
Frillingos S, Sahin-Toth M, Wu J, Kaback HR (1998) Cys-scanning mutagenesis: a novel approach to structure function relationships in polytopic membrane proteins. FASEB J 12:1281–1299
Fristedt U, Berhe A, Ensler K, Norling B, Persson BL (1996) Isolation and characterization of membrane vesicles of Saccharomyces cerevisiae harboring the high-affinity phosphate transporter. Arch Biochem Biophys 330:133–141
Fristedt U, Weinander R, Martinsson HS, Persson BL (1999a) Characterization of purified and unidirectionally reconstituted Pho84 phosphate permease of Saccharomyces cerevisiae. FEBS Lett 458:1–5
Fristedt U, Van Der Rest M, Poolman B, Konings WN, Persson BL (1999b) Studies of cytochrome c oxidase-driven H+_coupled phosphate transport catalyzed by the Saccharomyces cerevisiae Pho84 permease in coreconstituted vesicles. Biochemistry 38:16010–16015
Garciadeblas B, Rubino F, Quintero FJ, Banuelos MA, Haro R, Rodrigues-Navarro A (1993) Differential expression of two genes encoding isoforms of the ATPase involved in sodium efflux in Saccharomyces cerevisiae. Mol Gen Genet 236:363–368
Giots F, Donaton MCV, Thevelein JM (2003) Inorganic phosphate is sensed by specific phosphate carriers and acts in concert with glucose as a nutrient signal for activation of the protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol Microbiol 47:1163–1181
Gouffeau A, Aert R, Agostini-Carbone ML, et al (1997) The yeast genome directory. Nature 387 [Suppl]:1–105
Graschopf A, Stadler JA, Hoellerer MK, Eder S, Sieghardt M, Kohlwein SD, Schweyen RJ (2001). The yeast plasma membrane protein Alr1 controls Mg2+ homeostasis and is subject to Mg2+-dependent control of its synthesis and degradation. J Biol Chem 276:16216–16222
Gray NS, Wodicka L, Thunnissen AM, Norman TC, Kwon S, Espinoza FH, Morgan DO, Barnes G, LeClerc S, Meijer L, Kim SH, Lockhart DJ, Schultz PG (1998) Exploiting chemical libraries, structure, and genomics in the search for kinase inhibitors. Science 281:533–538
Grünewald M, Menaker D, Kanner BI (2002) Cysteine-scanning mutagenesis reveals a conformationally sensitive reentrant pore-loop in the glutamate transporter GLT-1. J Biol Chem 277:26074–26080
Haguenauer-Tsapis R, Nagy M, Ryter A (1986) A deletion that includes the segment coding for the signal peptidase cleavage site delays release of Saccharomyces cerevisiae acid phosphatase from the endoplasmic reticulum. Mol Cell Biol 6:723–729
Harold FM (1966) Inorganic polyphosphates in biology: structure, metabolism, and function. Bacteriol Rev 30:772–794
Henderson PJF (1993) The 12-transmembrane helix transporters. Curr Opin Cell Biol 5:708–721
Herrmann JM, Malkus P, Schekman R (1999) Out of the ER–outfitters, escorts and guides. Trends Cell Biol 9:5–7
Hicke L (1999) Gettin′ down with ubiquitin: turning off cell-surface receptors, transporters and channels. Trends Cell Biol 9:107–112
Hicke L, Riezman H (1996) Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell 84:277–287
Hicke L, Zanolari B, Riezman HJ (1998) Cytoplasmic tail phosphorylation of the alpha-factor receptor is required for its ubiquitination and internalization. J Cell Biol 141:349–358
Hirimburegama K, Durnez P, Keleman J, Oris E, Vergauwen R, Mergelsberg H, Thevelein JM (1992) Nutrient-induced activation of trehalase in nutrient-starved cells of the yeast Saccharomyces cerevisiae: cAMP is not involved as second messenger. J Gen Microbiol 138:2035–2043
Hirose E, Nakashima N, Sekiguchi T, Nishimoto T (1998) RagA is a functional homologue of the S. cerevisiae Gtr1p in the Ran/Gsp1-GTPase pathway. J Cell Sci 111:11–21
Hohmann S, Meacock PA (1998) Thiamin metabolism and thiamin diphosphate-dependent enzymes in the yeast Saccharomyces cerevisiae: genetic regulation. Biochim Biophys Acta 1385:201–219
Hope IA, Struhl K (1985) GCN4 protein, synthesized in vitro, binds HIS3 regulatory sequences: implications for general control of amino acid biosynthetic genes in yeast. Cell 43:177–188
Horak J, Wolf DH (1997) Catabolite inactivation of the galactose transporter in the yeast Saccharomyces cerevisiae: ubiquitination, endocytosis, and degradation in the vacuole. J Bacteriol 179:1541–1549
Huang D, Moffat J, Wilson WA, Moore L, Cheng C, Roach PJ, Andrews B (1998) Cyclin partners determine Pho85 protein kinase substrate specificity in vitro and in vivo: control of glycogen biosynthesis by Pcl8 and Pcl10. Mol Cell Biol 18:3289–3299
Huang S, Jeffery DA, Anthony MD, O′Shea EK (2001) Functional analysis of the cyclin-dependent kinase inhibitor Pho81 identifies a novel inhibitory domain. Mol Cell Biol 21:6695–6705
Jacobson L, Halmann M, Yariv J (1982) The molecular composition of the volutin granule of yeast. Biochem J 201:473–479
Jeffery DA, Springer M, King DS, O′Shea EK (2001) Multi-site phosphorylation of Pho4 by the cyclin-CDK Pho80–Pho85 is semi-processive with site preference. J Mol Biol 306:997–1010
Kaffman A, Herskowitz I, Tjian R, O′Shea EK (1994) Phosphorylation of the transcription factor PHO4 by a cyclin-CDK complex, PHO80–PHO85. Science 263:1153–1156
Kaffman A, Rank NM, O′Neill EM, Huang LS, O′Shea EK (1998a) The receptor Msn5 exports the phosphorylated transcription factor Pho4 out of the nucleus. Nature 396:482–486
Kaffman A, Rank NM, O′Shea EK (1998b) Phosphorylation regulates association of the transcription factor Pho4 with its import receptor Pse1/Kap121. Genes Dev 12:2673–2683
Kaneko Y, Toh-e A, Oshima Y (1982) Identification of the genetic locus for the structural gene and a new regulatory gene for the synthesis of repressible alkaline phosphatase in Saccharomyces cerevisiae. Mol Cell Biol 2:127–137
Kaneko Y, Tamai Y, Toh-e A, Oshima Y (1985) Transcriptional and post-translational control of PHO84 expression by PHO regulatory genes in Saccharomyces cerevisiae. Mol Cell Biol 5:248–252
Kaneko Y, Hayashi N, Toh-e A, Banno I, Oshima Y (1987) Structural characteristics of the PHO8 gene encoding repressible alkaline phosphatase in Saccharomyces cerevisiae. Gene 58:137–148
Kaneko Y, Toh-e A, Banno I, Oshima Y (1989) Molecular characterization of a specific p-nitrophenylphosphatase gene, PHO13, and its mapping by chromosome fragmentation in Saccharomyces cerevisiae. Mol Gen Genet 220:133–139
Karpichev IV, Cornivelli L, Small GM (2002) Multiple regulatory roles of a novel Saccharomyces cerevisiae protein, encoded by YOL002c, in lipid and phosphate metabolism. J Biol Chem 277:19609–19617
Kohler K, Forster IC, Stange G, Biber J, Murer H (2002) Identification of functionally important sites in the first intracellular loop of the NaPi-IIa cotransporter. Am J Physiol Ren Physiol 282:687–696
Komeili A, O′Shea EK (1999) Roles of phosphorylation sites in regulating activity of the transcription factor Pho4. Science 284:977–980
Komeili A, O′Shea EK (2000) Nuclear transport and transcription. Curr Opin Cell Biol 12:355–360
Kornberg A (1995) Inorganic polyphosphate: toward making a forgotten polymer unforgettable. J Bacteriol 177:491–496
Kornberg A, Rao NN, Ault-Riche D (1999) Inorganic polyphosphate: a molecule of many functions. Annu Rev Biochem 68:89–125
Krampe S, Stamm O, Hollenberg CP, Boles E (1998) Catabolite inactivation of the high-affinity hexose transporters Hxt6 and Hxt7 of Saccharomyces cerevisiae occurs in the vacuole after internalization by endocytosis. FEBS Lett 441:343–347
Krebs JE, Fry CJ, Samuels ML, Peterson CL (2000) Global role for chomatin remodeling enzymes in mitotic gene expression. Cell 102:587–598
Kruckeberg AL (1996) The hexose transporter family of Saccharomyces cerevisiae. Arch Microbiol 166:283–292
Kruckeberg AL, Ye L, Berden JA, Dam K van (1999) Functional expression, quantification and cellular localization of the Hxt2 hexose transporter of Saccharomyces cerevisiae tagged with the green fluorescent protein. Biochem J 339:299–307
Kulaev IS (1979) The biochemistry of inorganic polyphosphates. Wiley, New York
Kulaev I, Kulakovskaya T (2000) Polyphosphate and phosphate pump. Annu Rev Microbiol 54:709–734
Kulaev IS, Vagabov VM (1983) Polyphosphate metabolism in microorganisms. Adv Microb Physiol 24:83–171
Kulaev I, Vagabov V, Kulakovskaya T (1999) New aspects of polyphosphate metabolism and function. J Biosci Bioeng 88:111–129
Kumble KD, Kornberg A (1996) Endopolyphosphatases for long chain inorganic polyphosphate in yeast and mammals. J Biol Chem 271:27146–27151
Lagerstedt JO, Kruckeberg AL, Berden JA, Persson BL (2000) The yeast phosphate transporting system: regulated trafficking of the Pho84 phosphate transporter. In: Hohmann S, Nielsen S (eds) Molecular biology and physiology of water and solute transport: fundamental research and applied aspects. Kluwer/Plenum, New York, pp 405–414
Lagerstedt JO, Zvyagilskaya R, Pratt JR, Pattison-Granberg J, Kruckeberg AL, Berden JA, Persson BL (2002) Mutagenic and functional analysis of the C-terminus of Saccharomyces cerevisiae Pho84 phosphate transporter. FEBS Lett 526:31–37
Lamb TM, Xu W, Diamond A, Mitchell AP (2001) Alkaline response genes of Saccharomyces cerevisiae and their relationship to the RIM101 pathway. J Biol Chem 276:1850–1856
Lau WW, Schneider KR, O′Shea EK (1998) A genetic study of signaling processes for repression of PHO5 transcription in Saccharomyces cerevisiae. Genetics 150:1349–1359
Lau WT, Howson RW, Malkus P, Schekman R, O′Shea EK (2000) Pho86p, an endoplasmic reticulum (ER) resident protein in Saccharomyces cerevisiae, is required for ER exit of the high-affinity phosphate transporter Pho84p. Proc Natl Acad Sci USA 97:1107–1112
Lemire JM, Willcocks T, Halvorson HO, Bostian KA (1985) Regulation of repressible acid phosphatase gene transcription in Saccharomyces cerevisiae. Mol Cell Biol 5:2131–2141
Lenburg ME, O′Shea EK (1996) Signaling phosphate starvation. Trends Biochem Sci 21:383–387
Lichko LP, Kulakovskaia TV, Kulaev IS (1996) Characteristics of polyphosphatase activity of isolated mitochondria from Saccharomyces cerevisiae. Biokhimiya 61:1664–1671
Lichko L, Kulakovskaya T, Kulaev I (1998) Membrane-bound and soluble polyphosphatases of mitochondria of Saccharomyces cerevisiae: identification and comparative characterization. Biochim Biophys Acta 1372:153–162
Lichko LP, Kulakovskaya TV, Kulaev IS (2000) Purification and characterization of a soluble polyphosphatase from mitochondria of Saccharomyces cerevisiae. Biochemistry (Mosc) 65:355–360
Liu C, Yang Z, Yang J, Xia Z, Ao S (2000) Regulation of the yeast transcriptional factor PHO2 activity by phosphorylation. J Biol Chem 275:31972–31978
Martinez P, Persson BL (1998) Identification, cloning and characterization of a derepressible Na+-coupled phosphate transporter in Saccharomyces cerevisiae. Mol Gen Genet 258:628–638
Martinez P, Zvyagilskaya R, Allard P, Persson BL (1998) Physiological regulation of the derepressible phosphate transport in Saccharomyces cerevisiae. J Bacteriol 180:2253–2256
Measday V, Moore L, Ogas J, Tyers M, Andrews B (1994) The PCL2 (ORFD)–PHO85 cyclin-dependent kinase complex: a cell cycle regulator in yeast. Science 266:1391–1395
Measday V, Moore L, Retnakaran R, Lee J, Donoviel M, Neiman AM, Andrews B (1997) A family of cyclin-like proteins that interact with the Pho85 cyclin-dependent kinase. Mol Cell Biol 17:1212–1223
Mendoza I, Rubio F, Rodrigues-Navarro A, Pardo JM (1994) The protein phosphatase calcineurin is essential for NaCl tolerance of Saccharomyces cerevisiae. J Biol Chem 269:8792–8796
Morgan DO (1995) Principles of CDK regulation. Nature 374:131–134
Müller O, Bayer MJ, Peters C, Andersen JS, Mann M, Mayer A (2002) The Vtc proteins in vacuole fusion: coupling NSF activity to V(0) trans-complex formation. EMBO J 21:259–269
Münsterkötter M, Barbaric S, Horz W (2000) Transcriptional regulation of the yeast PHO8 promoter in comparison to the coregulated PHO5 promoter. J Biol Chem 275:22678–22685
Nakashima N, Noguchi E, Nishimoto T (1999) Saccharomyces cerevisiae putative G protein, Gtr1p, which forms complexes with itself and a novel protein designated as Gtr2p, negatively regulates the Ran/Gsp1p G protein cycle through Gtr2p. Genetics 152:853–867
Natarajan K, Meyer MR, Jackson BM, Slade D, Roberts C, Hinnebusch AG, Marton MJ (2001) Transcriptional profiling shows that Gcn4p is a master regulator of gene expression during amino acid starvation in yeast. Mol Cell Biol 21:4347–4368
Nelissen B, Mordant P, Jonniaux J-L, De Wachter R, Gouffeau A (1995) Phylogenetic classification of the major superfamily of membrane transport facilitator, as deduced from yeast genome sequencing. FEBS Lett 377:232–236
Nelson H, Nelson N (1990) Disruption of genes encoding subunits of the yeast vacuolar H(+)-ATPase causes conditional lethality. Proc Natl Acad Sci USA 87:3503–3507
Nigavekar SS, Tan YS, Cannon JF (2002) Glc8 is a glucose-repressible activator of Glc7 protein phosphatase-1. Arch Biochem Biophys 404:71–79
Nishimura H, Kawasaki Y, Kaneko Y, Nosaka K, Iwashima A (1992a) A positive regulatory gene, THI3, is required for thiamine metabolism in Saccharomyces cerevisiae. J Bacteriol 174:4701–4706
Nishimura H, Kawasaki Y, Kaneko Y, Nosaka K, Iwashima A (1992b) Cloning and characteristics of a positive regulatory gene, THI2 (PHO6), of thiamin biosynthesis in Saccharomyces cerevisiae. FEBS Lett 297:155–158
Norbeck J, Pahlman AK, Akhtar N, Blomberg A, Adler L (1996) Purification and characterization of two isoenzymes of dl-glycerol-3-phosphatase from Saccharomyces cerevisiae. Identification of the corresponding GPP1 and GPP2 genes and evidence for osmotic regulation of Gpp2p expression by the osmosensing mitogen-activated protein kinase signal transduction pathway. J Biol Chem 271:13875–13881
Nosaka K (1990) High affinity of acid phosphatase encoded by PHO3 gene in Saccharomyces cerevisiae for thiamin phosphates. Biochim Biophys Acta 1037:147–154
Ogawa N, Oshima Y (1990) Functional domains of a positive regulatory protein, pho4, for transcriptional control of the phosphatase regulon in Saccharomyces cerevisiae. Mol Cell Biol 10:2224–2236
Ogawa N, Noguchi K, Yamashita Y, Yasuhara T, Hayashi N, Youshida K, Oshima Y (1993) Promoter analysis of the PHO81 gene encoding a 134 kDa protein bearing ankyrin repeats in the phosphatase regulon of Saccharomyces cerevisiae. Mol Gen Genet 238:444–454
Ogawa N, Noguchi K, Sawai H, Yamashita Y, Yompakdee C, Oshima Y (1995) Functional domains of Pho81p, an inhibitor of Pho85p protein kinase, in the transduction pathway of Pi signals in Saccharomyces cerevisiae. Mol Cell Biol 15:997–1004
Ogawa N, DeRisi J, Brown PO (2000) New components of a system for phosphate accumulation and polyphosphate metabolism in Saccharomyces cerevisiae revealed by genomic expression analysis. Mol Biol Cell 11:4309–4321
O′Neill EM, Kaffman A, Jolly ER, O′Shea EK (1996) Regulation of PHO4 nuclear localization by the PHO80–PHO85 cyclin-CDK complex. Science 271:209–212
Oshima Y (1997) The phosphatase system in Saccharomyces cerevisiae. Genes Genet Syst 72:323–334
Oshima Y, Ogawa N, Harashima S (1996) Regulation of phosphatase synthesis in Saccharomyces cerevisiae. Genetics 149:1707–1715
Özcan S, Dover J, Rosenwald AG, Wölfl S, Johnston M (1996) Two glucose transporters in Saccharomyces cerevisiae are glucose sensors that generate a signal for induction of gene expression. Proc Natl Acad Sci USA 93:12428–12432
Pao SS, Paulsen IT, Saier MH Jr (1998) Major facilitator superfamily. Microbiol Mol Biol Rev 62:1–34
Pattison-Granberg J, Persson BL (2000) Regulation of cation-coupled high-affinity phosphate uptake in the yeast Saccharomyces cerevisiae. J Bacteriol 182:5017–5019
Patton-Vogt JL, Henry SA (1998) GIT1, a gene encoding a novel transporter for glycerophosphoinositol in Saccharomyces cerevisiae. Genetics 149:1707–1715
Persson BL, Petersson J, Fristedt U, Weinander R, Berhe A, Pattison J (1999) Phosphate permeases of Saccharomyses cerevisiae: structure, function and regulation. Biochim Biophys Acta 1422:255–272
Petersson J, Pattison J, Kruckeberg AL, Berden JA, Persson BL (1999) Intracellular localization of an active green fluorescent protein-tagged Pho84 phosphate permease in Saccharomyces cerevisiae. FEBS Lett 462:37–42
Pick U, Bental M, Chitlaru E, Weiss M (1990) Polyphosphate hydrolysis—a protective mechanism against alkaline stress? FEBS Lett 274:15–18
Plankert U, Purwin C, Holzer H (1991) Yeast fructose-2,6-bisphosphate 6-phosphatase is encoded by PHO8, the gene for nonspecific repressible alkaline phosphatase. Eur J Biochem 196:191–196
Rao NN, Liu S, Kornberg A (1998) Inorganic polyphosphate in Escherichia coli: the phosphate regulon and the stringent response. J Bacteriol 180:2186–2193
Raths S, Rohrer J, Crausaz F, Riezman H (1993) end3 and end4: two mutants defective in receptor-mediated and fluid-phase endocytosis in Saccharomyces cerevisiae. J Cell Biol 120:55–65
Rechsteiner M, Rogers SW (1996) PEST sequences and regulation by proteolysis. Trends Biochem Sci 21:267–271
Reusch RN, Sadoff HL (1988) Putative structure and function of a poly-beta-hydroxybutyrate/calcium phosphate channel in bacterial plasma membranes. Proc Natl Acad Sci USA 85:4176–4180
Riballo E, Herweijer M, Wolf DH, Lagunas R (1995) Catabolite inactivation of the yeast maltose transporter occurs in the vacuole after internalization by endocytosis. J Bacteriol 177:5622–5627
Roomans GM, Blasco F, Borst-Pauwels GW (1977) Cotransport of phosphate and sodium by yeast. Biochim Biophys Acta 467:65–71
Rotin D, Staub O, Haguenauer-Tsapis R (2000) Ubiquitination and endocytosis of plasma membrane proteins: role of Nedd4/Rsp5p family of ubiquitin–protein ligases. J Membr Biol 176:1–17
Rubin RA, Levy SB, Heinrikson RL, Kézdy FJ (1990) Gene duplication in the evolution of the two complementing domains of gram-negative bacterial tetracycline efflux protein. Gene 87:7–13
Rudolph H, Hinnen A (1987) The yeast PHO5 promoter: phosphate-control elements and sequences mediating mRNA start-site selection. Proc Natl Acad Sci USA 84:1340–1344
Saier MH Jr (2000) A functional–phylogenetic classification system for transmembrane solute transporters. Microbiol Mol Biol Rev 64:354–411
Salaün C, Rodrigues P, Heard JM (2001) Transmembrane topology of PiT-2 phosphate transporter-retrovirus receptor. J Virol 75:5584–5592
Sato M, Mueckler M (1999) A conserved amino acid motif (R-X-G-R-R) in the Glut1 glucose transporter is an important determinant of membrane topology. J Biol Chem 274:24721–24725
Schneider KR, Smith RL, O′Shea EK (1994) Phosphate-regulated inactivation of the kinase PHO80–PHO85 by the CDK inhibitor PHO81. Science 266:122–126
Schuddemat J, Boo R de, Leeuwen CC van, Broek PJ van den, Steveninck J van (1989) Polyphosphate synthesis in yeast. Biochim Biophys Acta 1010:191–198
Schurr A, Yagil E (1971) Regulation and characterization of acid and alkaline phosphatase in yeast. J Gen Microbiol 65:291–303
Sedgwick SG, Smerdon SJ (1999) The ankyrin repeat: a diversity of interactions on a common structural framework. Trends Biochem Sci 24:311–316
Seedorf M, Silver PA (1997) Importin/karyopherin protein family members required for mRNA export from the nucleus. Proc Natl Acad Sci USA 94:8590–8595
Sengstag C, Hinnen A (1987) The sequence of the Saccharomyces cerevisiae gene PHO2 codes for a regulatory protein with unusual amino acid composition. Nucleic Acids Res 15:233–246
Serrano R (1996) Salt tolerance in plants and microorganisms: toxicity targets and defence responses. Int Rev Cytol 165:1–52
Serrano R, Ruiz A, Bernal D, Chambers JR, Arino J (2002) The transcriptional response to alkaline pH in Saccharomyces cerevisiae: evidence for calcium-mediated signalling. Mol Microbiol 46:1319–1333
Sethuraman A, Rao NN, Kornberg A (2001) The endopolyphosphatase gene: essential in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 98:8542–8547
Shao D, Creasy CL, Bergman LW (1998) A cysteine residue in helix II of the bHLH domain is essential for homodimerization of the yeast transcription factor Pho4p. Nucleic Acids Res 26:710–714
Shemer R, Meimoun A, Holtzman T, Kornitzer D (2002) Regulation of the transcription factor gcn4 by pho85 cyclin pcl5. Mol Cell Biol 22:5395–5404
Shimizu T, Toumoto A, Ihara K, Shimizu M, Kyogoku Y, Ogawa N, Oshima Y, Hakoshima T (1997) Crystal structure of PHO4 bHLH domain-DNA complex: flanking base recognition. EMBO J 16:4689–4697
Steensma HY de, Jonge P de, Kaptein A, Kaback DB (1989) Molecular cloning of chromosome I DNA from Saccharomyces cerevisiae: localization of a repeated sequence containing an acid phosphatase gene near a telomere of chromosome I and chromosome VIII. Curr Genet 16:131–137
Steger DJ, Haswell ES, Miller AL, Wente SR, O′Shea EK (2003) Regulation of chromatin remodeling by inositol polyphosphatases. Science 299:114–116
Stolz LE, Kuo WJ, Longchamps J, Sekhon MK, York JD (1998) INP51, a yeast inositol polyphosphate 5-phosphatase required for phosphatidylinositol 4,5-bisphosphate homeostasis and whose absence confers a cold-resistant phenotype. J Biol Chem 273:11852–11861
Sudarsanam P, Iyer VR, Brown PO, Winston F (2000) Whole-genome expression analysis of snf/swi mutants of Saccharomyces cerevisiae. Proc Natl Acad Sci USA 97:3364–3369
Tamai Y, Toh-e A, Oshima Y (1985) Regulation of inorganic phosphate transport systems in Saccharomyces cerevisiae. J Bacteriol 164:964–968
Tan YS, Morcos PA, Cannon JF (2003) Pho85 phosphorylates the Glc7 protein phosphatase regulator Glc8 in vivo. J Biol Chem 278:278147–278153
Tijssen JP, Van Steveninck J (1984) Detection of a yeast polyphosphate fraction localized outside the plasma membrane by the method of phosphorus-31 nuclear magnetic resonance. Biochem Biophys Res Commun 119:447–451
Toh-e A, Kakimoto S (1975) Genes coding for the structure of the acid phosphatases in Saccharomyces cerevisiae. Mol Gen Genet 143:65–70
Toh-e A, Shimauchi T (1986) Cloning and sequencing of the PHO80 gene and CEN15 of Saccharomyces cerevisiae. Yeast 2:129–139
Toh-e A, Ueda Y, Kakimoto S-I, Oshima Y (1973) Isolation and characterization of acid phosphatase mutants in Saccharomyces cerevisiae. J Bacteriol 113:727–738
Toh-e A, Tanaka K, Uesono Y, Wickner RB (1988) PHO85, a negative regulator of the PHO system, is a homolog of the protein kinase gene, CDC28, of Saccharomyces cerevisiae. Mol Gen Genet 214:162–164
Trilisenko LV, Vagabov VM, Kulaev IS (2002) The content and chain length of polyphosphates from vacuoles of Saccharomyces cerevisiae VKM Y-1173. Biochemistry (Mosc) 67:592–596
Tsutsumi K, Munekata M, Shiba T (2000) Involvement of inorganic polyphosphate in expression of SOS genes. Biochim Biophys Acta 1493:73–81
Uesono Y, Tanaka K, Toh-e A (1987) Negative regulators of the PHO system in Saccharomyces cerevisiae: isolation and structural characterization of PHO85. Nucleic Acids Res 15:10299–10309
Uesono Y, Tokai M, Tanaka K, Tohe A (1992) Negative regulators of the PHO system of Saccharomyces cerevisiae: characterization of PHO80 and PHO85. Mol Gen Genet 231:426–432
Urech K, Durr M, Boller T, Wiemken A, Schwencke J (1978) Localization of polyphosphate in vacuoles of Saccharomyces cerevisiae. Arch Microbiol 116:275–278
Vagabov VM, Trilisenko LV, Kulaev IS (2000) Dependence of inorganic polyphosphate chain length on the orthophosphate content in the culture medium of the yeast Saccharomyces cerevisiae. Biochemistry (Mosc) 65:349–54
Van der Rest ME, Kamminga AH, Nakano A, Anraku Y, Poolman B, Konings WN (1995) The plasma membrane of Saccharomyces cerevisiae: structure, function, and biogenesis. Microbiol Rev 59:304–322
Van Dien SJ, Keasling JD (1999) Effect of polyphosphate metabolism on the Escherichia coli phosphate-starvation response. Biotechnol Prog 15:587–593
Venter U, Hörz W (1989) The acid phosphatase genes PHO10 and PHO11 in S. cerevisiae are located at the telomeres of chromosomes VIII and I. Nucleic Acids Res 17:1353–1369
Venter U, Svaren J, Schmitz J, Schmid A, Hörz W (1994) A nucleosome precludes binding of the transcription factor Pho4 in vivo to a critical target site in the PHO5 promoter. EMBO J 13:4848–4855
Walker GM (1998). Yeast physiology and biotechnology. Wiley, New York, pp 81–82
Walker JE, Saraste M, Runswick MH, Gay NJ (1982) Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J 1:945–951
Wang Z, Wilson WA, Fujino MA, Roach PJ (2001) The yeast cyclins Pc16p and Pc17p are involved in the control of glycogen storage by the cyclin-dependent protein kinase Pho85p. FEBS Lett 506:277–280
Wood HG, Clark JE (1988) Biological aspects of inorganic polyphosphates. Annu Rev Biochem 57:235–260
Wu WH, Hampsey M (1999) An activation-specific role for transcription factor TFIIB in vivo. Proc Natl Acad Sci USA 96:2764–2769
Wurst H, Kornberg A (1994) A soluble exopolyphosphatase of Saccharomyces cerevisiae. Purification and characterization. J Biol Chem 269:10996–11001
Wurst H, Shiba T, Kornberg A (1995) The gene for a major exopolyphosphatase of Saccharomyces cerevisiae. J Bacteriol 177:898–906
Wykoff DD, O′Shea EK (2001) Phosphate transport and sensing in Saccharomyces cerevisiae. Genetics 159:1491–1499
Yale J, Bohnert HJ (2001) Transcript expression in Saccharomyces cerevisiae at high salinity. J Biol Chem 276:15996–16007
Yompakdee C, Bun-ya M, Shikata K, Ogawa N, Harashima S, Oshima Y (1996a) A putative new membrane protein, Pho86p, in the inorganic phosphate uptake system of Saccharomyces cerevisiae. Gene 171:41–47
Yompakdee C, Ogawa N, Harashima S, Oshima Y (1996b) A putative membrane protein, Pho88p, involved in inorganic phosphate transport in Saccharomyces cerevisiae. Mol Gen Genet 251:580–590
Yoshida K, Kuromitsu Z, Ogawa N, Oshima Y (1989) Mode of expression of the positive regulatory genes PHO2 and PHO4 of the phosphatase regulon in Saccharomyces cerevisiae. Mol Gen Genet 217:31–39
Zhu H, Riggs AF (1992) Yeast flavohemoglobin is an ancient protein related to globins and a reductase family. Proc Natl Acad Sci USA 89:5015–5019
Acknowledgements
Work in the authors' laboratories was supported by research grants from the Human Frontier Science Organization and the Swedish Royal Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Hohmann
Rights and permissions
About this article
Cite this article
Persson, B.L., Lagerstedt, J.O., Pratt, J.R. et al. Regulation of phosphate acquisition in Saccharomyces cerevisiae . Curr Genet 43, 225–244 (2003). https://doi.org/10.1007/s00294-003-0400-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-003-0400-9