Abstract
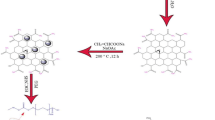
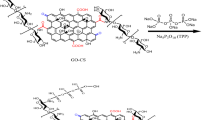
Research has commonly utilized graphene-based drug delivery systems for a long time to achieve effective cancer treatment. In the present study, doxorubicin (DOX) and selective estrogen receptor modulator tamoxifen (TAM) anticancer drugs used in breast cancer treatment were bound to a graphene oxide (GO)-based and folic acid (FA)-targeted nanocarrier system that was made biocompatible with chitosan (CS). To this end, graphene oxide synthesis was primarily carried out by employing the modified Hummer's method, and then FA and CS were loaded on GO to obtain a targeted and biocompatible carrier The characterization of the obtained conjugate was performed by X-ray diffraction analysis, Fourier transform infrared spectroscopy, UV-visible spectrophotometry, scanning electron microscopy, and zeta potential analysis. The zeta potential values of all samples were checked and all of them have a zeta potential above the stability value of ± 25 mV. GO-CS-FA has the highest zeta potential of 68.8 mV. The graphene oxide-chitosan-folic acid-tamoxifen-doxorubicin (GO-CS-FA-TAM-DOX) nanocarrier-based drug displayed a pH-dependent drug release. The drug release profile from these systems was researched in two pH buffer solutions prepared as acidic (pH 5.8) and physiological (pH 7.4). The characterization analyses showed that the drugs bound successfully to the targeted delivery system. The drug release analyses demonstrated that GO-CS-FA-TAM-DOX was released better in the acidic (pH 5.8) medium compared to the physiological (pH 7.4) medium after 24 h.







Similar content being viewed by others
References
van der Zee J (2002) Heating the patient: a promising approach? Ann Oncol 13(8):1173–1184
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69(1):7–34
Thomadaki H, Scorilas A (2008) Molecular profile of breast versus ovarian cancer cells in response to treatment with the anticancer drugs cisplatin, carboplatin, doxorubicin, etoposide and taxol. Biol Chem 389(11):1427–1434
Bronchud MH, Howell A, Crowther D, Hopwood P, Souza L, Dexter TM (1989) The use of granulocyte colony-stimulating factor to increase the intensity of treatment with doxorubicin in patients with advanced breast and ovarian cancer. Br J Cancer 60(1):121–125
Ikeda M et al (2011) The radiotherapy with methotrexate, vinblastine, doxorubicin, and cisplatin treatment is an effective therapeutic optionin patients with advanced or metastatic bladder cancer. J Radiat Res 52(5):674–679
Huober J, Schoch O, Templeton A, Spirig C, Thürlimann B (2010) Interstitial pneumonitis after treatment with bevacizumab and pegylated liposomal doxorubicin in a patient with metastatic breast cancer. Chemotherapy 56(1):69–70
Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L (2004) Anthracyclines: molecular advances and pharmacologie developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56(2):185–229
Kim D, Lee ES, Oh KT, Gao Z, Bae YH (2008) Doxorubicin-loaded polymeric micelle overcomes multidrug resistance of cancer by double-targeting folate receptor and early endosomal pH. Small 4(11):2043–2050
Betancourt T, Brown B, Brannon-Peppas L (2007) Doxorubicin-loaded PLGA nanoparticles by nanoprecipitation: preparation, characterization and in vitro evaluation. Nanomedicine 2(2):219–232
Chang M (2012) Tamoxifen resistance in breast cancer. Biomol Ther 20(3):256–267
Zhang L, Lu J, Jin Y, Qiu L (2014) Folate-conjugated beta-cyclodextrin-based polymeric micelles with enhanced doxorubicin antitumor efficacy. Colloids Surf B Biointerfaces 122:260–269
Patel HK, Bihani T (2018) Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol Ther 186:1–24
How CW, Rasedee A, Manickam S, Rosli R (2013) Tamoxifen-loaded nanostructured lipid carrier as a drug delivery system: characterization, stability assessment and cytotoxicity. Colloids Surf B Biointerfaces 112:393–399
Hertl M, Cosimi AB (2005) Liver transplantation for malignancy. Oncologist 10(4):269–281
Lazzeroni M, Serrano D, Dunn BK, Heckman-Stoddard BM, Lee O, Khan S, Decensi A (2012) Oral low dose and topical tamoxifen for breast cancer prevention: modern approaches for an old drug. Breast Cancer Res 14(5):1–11
Vivek R, Nipun Babu V, Thangam R, Subramanian KS, Kannan S (2013) pH-responsive drug delivery of chitosan nanoparticles as Tamoxifen carriers for effective anti-tumor activity in breast cancer cells. Colloids Surf B Biointerfaces 111:117–123
Chuang P-Y, Huang C, Huang H-C (2013) The use of a combination of tamoxifen and doxorubicin synergistically to induce cell cycle arrest in BT483 cells by down-regulating CDK1, CDK2 and cyclin D expression. J Pharm Technol Drug Res 2(1):12
Youlden DR, Cramb SM, Dunn NAM, Muller JM, Pyke CM, Baade PD (2012) The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol 36(3):237–248
Kateb B et al (2011) Nanoplatforms for constructing new approaches to cancer treatment, imaging, and drug delivery: what should be the policy? Neuroimage 54(SUPPL.1):1
He Z, Zhang Z, Bi S (2020) Nanoparticles for organic electronics applications. Mater Res Express 7(1):012004
Sousa De Almeida M, Susnik E, Drasler B, Taladriz-Blanco P, Petri-Fink A, Rothen-Rutishauser B (2021) Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem Soc Rev 50(9):5397–5434
Kazempour M, Namazi H, Akbarzadeh A, Kabiri R (2019) Synthesis and characterization of PEG-functionalized graphene oxide as an effective pH-sensitive drug carrier. Artif Cells Nanomed Biotechnol 47(1):90–94
Gao X, Li L, Cai X, Huang Q, Xiao J, Cheng Y (2021) Targeting nanoparticles for diagnosis and therapy of bone tumors: opportunities and challenges. Biomaterials 265:120404
Goenka S, Sant V, Sant S (2014) Graphene-based nanomaterials for drug delivery and tissue engineering. J Control Release 173(1):75–88
Swain AK, Pradhan L, Bahadur D (2015) Polymer stabilized Fe3O4-graphene as an amphiphilic drug carrier for thermo-chemotherapy of cancer. ACS Appl Mater Interfaces 7(15):8013–8022
Zare EN, Makvandi P, Ashtari B, Rossi F, Motahari A, Perale G (2020) Progress in conductive polyaniline-based nanocomposites for biomedical applications: a review. J Med Chem 63(1):1–22
Ulery BD, Nair LS, Laurencin CT (2011) Biomedical applications of biodegradable polymers. J Polym Sci Part B Polym Phys 49(12):832–864
Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R (2007) Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2(12):751–760
Wu J (2021) The enhanced permeability and retention (EPR) effect: the significance of the concept and methods to enhance its application. J Pers Med 11(8):771
Byrne JD, Betancourt T, Brannon-Peppas L (2008) Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev 60(15):1615–1626
Varshosaz J, Hassanzadeh F, Sadeghi H, Khadem M (2012) Galactosylated nanostructured lipid carriers for delivery of 5-FU to hepatocellular carcinoma. J Liposome Res 22(3):224–236
Chen YW, Su YL, Hu SH, Chen SY (2016) Functionalized graphene nanocomposites for enhancing photothermal therapy in tumor treatment. Adv Drug Deliv Rev 105:190–204
Gazzi A et al (2019) Photodynamic therapy based on graphene and MXene in cancer theranostics. Front Bioeng Biotechnol 7:295
Pei X, et al. PEGylated nano-graphene oxide as a nanocarrier for delivering mixed anticancer drugs to improve anticancer activity, nature.com
Veclani D, Tolazzi M, Melchior A (2020) Molecular interpretation of pharmaceuticals’ adsorption on carbon nanomaterials: theory meets experiments. Processes 8(6):642
Aliyev E, Filiz V, Khan MM, Lee YJ, Abetz C, Abetz V (2019) Structural Characterization of graphene oxide: surface functional groups and fractionated oxidative debris. Nanomater 9(8):1180
Eckhart KE, Holt BD, Laurencin MG, Sydlik SA (2019) Covalent conjugation of bioactive peptides to graphene oxide for biomedical applications. Biomater Sci 7(9):3876–3885
Tabish TA et al (2019) Graphene oxide-based targeting of extracellular cathepsin D and Cathepsin L as a novel anti-metastatic enzyme cancer therapy. Cancers (Basel) 11(3):319
Gu Z, Zhu S, Yan L, Zhao F, Zhao Y (2019) Graphene-based smart platforms for combined cancer therapy. Adv Mater 31(9):1800662
Tadyszak K, Wychowaniec JK, Litowczenko J Biomedical applications of graphene-based structures
Tas A, Keklikcioglu Cakmak N (2021) Synthesis of PEGylated nanographene oxide as a nanocarrier for docetaxel drugs and anticancer activity on prostate cancer cell lines. Hum Exp Toxicol 40(1):172–182
Deb A, Andrews NG, Raghavan V (2018) Natural polymer functionalized graphene oxide for co-delivery of anticancer drugs: in-vitro and in-vivo. Int J Biol Macromol 113:515–525
Feng W, Wang Z Biomedical applications of chitosan-graphene oxide nanocomposites
Pooresmaeil M, Namazi H (2022) “Chitosan based nanocomposites for drug delivery application. Nanotechnology for biomedical applications. Springer, Singapore, pp 135–201
Khan NK, Azad TA, Fuloria AK, Nawaz S, Subramaniyan A, Akhlaq V et al (2021) Chitosan-coated 5-fluorouracil incorporated emulsions as transdermal drug delivery matrices. Polym (Basel) 13(19):33
Pooresmaeil M, Namazi H (2020) Facile preparation of pH-sensitive chitosan microspheres for delivery of curcumin; characterization, drug release kinetics and evaluation of anticancer activity. Int J Biol Macromol 162:501–511
Farhoudian S, Yadollahi M, Namazi H (2016) Facile synthesis of antibacterial chitosan/CuO bio-nanocomposite hydrogel beads. Int J Biol Macromol 82:837–843
Keklikcioğlu Çakmak N, Küçükyazıcı M, Eroğlu A (2019) Synthesis and stability analysis of folic acid-graphene oxide nanoparticles for drug delivery and targeted cancer therapies. Int Adv Res Eng J 03(02):81–85
Schnoell J, Jank BJ, Kadletz-Wanke L, Stoiber S, Gurnhofer E, Schlederer M, Heiduschka G, Kenner L (2022) Protein expression of folate receptor alpha in adenoid cystic carcinoma of the head and neck. OncoTargets Ther 15:531
Sun C, Sze R, Zhang M (2006) Folic acid-PEG conjugated superparamagnetic nanoparticles for targeted cellular uptake and detection by MRI. J Biomed Mater Res Part A 78(3):550–557
Oh JK, Park JM (2011) Iron oxide-based superparamagnetic polymeric nanomaterials: design, preparation, and biomedical application. Prog Polym Sci (Oxford) 36(1):168–189
Deb A, Vimala R (2018) Camptothecin loaded graphene oxide nanoparticle functionalized with polyethylene glycol and folic acid for anticancer drug delivery. J Drug Deliv Sci Technol 43(November):333–342
Qin XC, Guo ZY, Liu ZM, Zhang W, Wan MM, Yang BW (2013) Folic acid-conjugated graphene oxide for cancer targeted chemo-photothermal therapy. J Photochem Photobiol B Biol 120:156–162
Low PS, Henne WA, Doorneweerd DD (2008) Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc Chem Res 41(1):120–129
Marcano DC et al (2010) Improved synthesis of graphene oxide. ACS Nano 4(8):4806–4814
P Ghasemiyeh, S Mohammadi-Samani, Polymers blending as release modulating tool in drug delivery
Arkaban H et al (2022) Polyacrylic acid nanoplatforms: antimicrobial, tissue engineering, and cancer theranostic applications. Polymers 14:1259
Jain S, Rathi VV, Jain AK, Das M, Godugu C (2012) Folate-decorated PLGA nanoparticles as a rationally designed vehicle for the oral delivery of insulin. Nanomedicine 7(9):1311–1337
Mortazavi N, Heidari M, Rabiei Z, Enferadi ST, Monazzah M (2021) Loading harmine on nanographene changes the inhibitory effects of free harmine against MCF-7 and fibroblast cells. Med Chem Res 30(5):1108–1116
Xie BX et al (2022) Folic acid-modified metal-organic framework carries CPT and DOX for cancer treatment. J Solid State Chem 306:122803
Hosseini SM et al (2022) Designing chitosan nanoparticles embedded into graphene oxide as a drug delivery system. Polym Bull 79(1):541–554
Lee H et al (2022) Hypoxia-responsive nanomedicine to overcome tumor microenvironment-mediated resistance to chemo-photodynamic therapy. Mater Today Adv 14:100218
Cafaggi S et al (2007) Preparation and evaluation of nanoparticles made of chitosan or N-trimethyl chitosan and a cisplatin-alginate complex. J Control Release 121(1–2):110–123
Park JH, Saravanakumar G, Kim K, Kwon IC (2010) Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv Drug Deliv Rev 62(1):28–41
Chun CJ, Lee SM, Kim SY, Yang HK, Song SC (2009) Thermosensitive poly(organophosphazene)-paclitaxel conjugate gels for antitumor applications. Biomaterials 30(12):2349–2360
Honary S, Zahir F (2013) Effect of zeta potential on the properties of nano-drug delivery systems - a review (part 1). Trop J Pharm Res 12(2):255–264
TM Magne, et al. (2021) Graphene and its derivatives: understanding the main chemical and medicinal chemistry roles for biomedical applications, no. 0123456789. Springer Berlin Heidelberg
De Sousa M, De Luna LAV, Fonseca LC, Giorgio S, Alves OL (2018) Folic-acid-functionalized graphene oxide nanocarrier: synthetic approaches, characterization, drug delivery study, and antitumor screening. ACS Appl Nano Mater 1:922–932
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Keklikcioglu Cakmak, N., Eroglu, A. Doxorubicin and tamoxifen loaded graphene oxide nanoparticle functionalized with chitosan and folic acid for anticancer drug delivery. Polym. Bull. 80, 2171–2185 (2023). https://doi.org/10.1007/s00289-022-04549-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04549-9