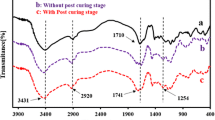
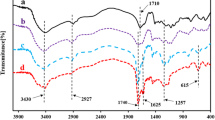
Abstract
Herein, poly (acrylic acid) (PAA) microgels were synthesized via alcohol type cross-linked by a free radical precipitation polymerization approach. At the first time, 1,6-hexanediol (1–6 diol), trimethylolpropane (TMP), and pentaerythritol (PEN) were selected as multifunctional cross- linking agent to synthesize cross-linked poly(acrylic acid) microgels. Alcohol type cross-linking agents can connect the PAA chains. The cross-linking reaction takes place due to reaction between hydroxyl groups of various cross-linkers and carboxyl groups of PAA chains. All of the hydroxyl groups do not participate in the reaction with acid groups of polymer chains through the polymerization stage; therefore, unreacted hydroxyl groups will react through sample drying (post-curing stage). The influence of cross-linker functionality and its concentration on various properties like swelling capacity, gel content, Tg (glass transition temperature), and rheological behavior were examined. The PAA microgels prepared via this cross-linking approach were compared to properties of microgels synthesized by epoxy type and vinyl type cross-linking agents in the previous studies. As a result, synthesized microgels via novel mechanisms have higher properties (for example, rheological and thermal properties) than that of PAA microgels prepared via the conventional mechanism. These behaviors can be due to decreasing \(\overline{Mc}\)(average molecular weight of two successive cross-links) in the polymeric network by utilizing new cross-linkers.











Similar content being viewed by others
References
Bin HY, Hashim S, Rahman WAWA (2017) Synthesis of polymeric nano/microgels: a review. J Polym Res 24:134
Begum R, Farooqi ZH, Khan SR (2016) Poly(N-isopropylacrylamide-acrylic acid) copolymer microgels for various applications: a review. Int J Polym Mater Polym Biomater 65:841–852
Rp U (2000) Temperature-sensitive aqueous microgels. Adv Colloid Interface Sci 85:1–33
Wu S, Dzubiella J, Kaiser J et al (2012) Thermosensitive Au-PNIPA yolk-shell nanoparticles with tunable selectivity for catalysis. Angew Chemie - Int Ed 51:2229–2233
Khan SR, Farooqi ZH, Ajmal M et al (2013) Synthesis, characterization, and silver nanoparticles fabrication in N-isopropylacrylamide-Based polymer microgels for rapid degradation of p-nitrophenol. J Dispers Sci Technol 34:1324–1333
Ngai T, Behrens SH, Auweter H (2005) Novel emulsions stabilized by pH and temperature sensitive microgels. Chem Commun 3:331. https://doi.org/10.1039/b412330a
Grabstain V, Bianco-Peled H (2003) Mechanisms controlling the temperature-dependent binding of proteins to poly(N-isopropylacrylamide) Microgels. Biotechnol Prog 19:1728–1733
Raemdonck K, Braeckmans K, Demeester J, De Smedt SC (2014) Merging the best of both worlds: hybrid lipid-enveloped matrix nanocomposites in drug delivery. Chem Soc Rev 43:444–472
Islam MR, Gao Y, Li X, Serpe MJ (2014) Responsive polymers for biosensing and protein delivery. J Mater Chem B 2:2444–2451
Suzuki D, Horigome K, Kureha T et al (2017) Polymeric hydrogel microspheres: design, synthesis, characterization, assembly and applications. Polym J 49:695–702
Hellweg T (2013) Responsive core-shell microgels: Synthesis, characterization, and possible applications. J Polym Sci Part B Polym Phys 51:1073–1083
Watanabe T, Kobayashi C, Song C et al (2016) Impact of spatial distribution of charged groups in core poly(N-isopropylacrylamide)-based microgels on the resultant composite structures prepared by seeded emulsion polymerization of styrene. Langmuir 32:12760–12773
Kumar Meena L, Rather H, Kedaria D, Vasita R (2020) Polymeric microgels for bone tissue engineering applications–a review. Int J Polym Mater Polym Biomater 69:381–397
Borro BC, Nordström R, Malmsten M (2020) Microgels and hydrogels as delivery systems for antimicrobial peptides. Colloids Surfaces B Biointerfaces 187:110835
Barry BW, Meyer MC (1979) The rheological properties of carbopol gels II. Oscillatory properties of carbopol gels. Int J Pharm 2:27–40
Liu J, Ran Q, Miao C, Zhou D (2011) Synthesis and characterization of comb-like copolymer dispersant with methoxy poly ( ethylene oxide ) side chains synthesis and characterization of comb-like copolymer dispersant with methoxy poly (ethylene oxide) side chains. Polym Plast Technol Eng 50:59–66
Bouhendi H, Haddadi-asl V, Rafizadeh M (2009) Effects of non-solvent type and purification process on precipitation polymerization of acrylic acid in organic media. Iran Polym J 18:777–787
Pourjavadi A, Kurdtabar M (2007) Collagen-based highly porous hydrogel without any porogen: synthesis and characteristics. Eur Polym J 43:877–889
Seiffert S (2014) Sensitive microgels as model colloids and microcapsules. J Polym Sci Part A Polym Chem 52:435–449
Kohestanian M, Bouhendi H (2016) Novel cross-linking mechanism with diol type cross-linkers, to prepare PAA Microgels via precipitation polymerization method. Polym–Plast Technol Eng 55:463–474
Bonham JA, Faers MA, Van Duijneveldt JS (2014) Non-aqueous microgel particles: synthesis, properties and applications. Soft Matter 10:9384–9398
Nur H, Snowden MJ, Cornelius VJ et al (2009) Colloidal microgel in removal of water from biodiesel. Colloids Surf A Physicochem Eng Asp 335:133–137
Camli ST, Buyukserin F, Yavuz MS, Budak GG (2010) Fine-tuning of functional poly(methylmethacrylate) nanoparticle size at the sub-100nm scale using surfactant-free emulsion polymerization. Colloids Surf A Physicochem Eng Asp 366:141–146
Kobayashi H, Shimizu I, Nakazawa M et al (1969) The photochromism of evaporated photospiran. Bull Chem Soc Jpn 42:2735–2735. https://doi.org/10.1246/bcsj.42.2735
Nie L, Jiang W, Yang W, et al (2005). Preparation of acrylic microgels by modified microemulsion polymerization and phase inversion. J Macromol Sci - Pure Appl Chem 42 A:623–631.
Pich A, Richtering W (2010) Microgels by precipitation polymerization: synthesis, characterization, and functionalization. Adv Polym Sci 234:1–37
Pardeshi S, Singh SK (2016) Precipitation polymerization: a versatile tool for preparing molecularly imprinted polymer beads for chromatography applications. RSC Adv 6:23525–23536
El-Aassar MR, Masoud MS, Elkady MF, Elzain AA (2018) Synthesis, optimization, and characterization of poly (Styrene-co-Acrylonitrile) copolymer prepared via precipitation polymerization. Adv Polym Technol 37:2021–2029
Chen-Jolly H, Guillot P, Mignard E (2018) Supercritical continuous precipitation polymerization of acrylic acid in a droplet-based millifluidic device. Chem Eng J 334:389–399
Nakano T, Saito N, Minami H (2020) Preparation of cross-linked monodisperse poly(acrylic acid) particles by precipitation polymerization. Langmuir 36:11957–11962
Cho S-H, Kim Y-J (2009) Synthesis of P(PEGMA-co-PBMA) microgels by precipitation polymerization in polymer solution. J Korea Acad Coop Soc 10:852–856
Goh ECC, Stöver HDH (2002) Cross-linked poly(methacrylic acid-co-poly(ethylene oxide) methyl ether methacrylate) microspheres and microgels prepared by precipitation polymerization: a morphology study. Macromolecules 35:9983–9989
Jong L (2020) Poly(acrylic acid) grafted soy carbohydrate as thickener for waterborne paints. Mater Today Commun 23:100882. https://doi.org/10.1016/j.mtcomm.2019.100882
Brady J, Dürig T, Lee PI, Li J-X (2017) Polymer Properties and Characterization. In: Developing Solid Oral Dosage Forms. Elsevier, 181–223.
R. Varges P, M. Costa C, S. Fonseca B et al (2019) Rheological characterization of carbopol® dispersions in water and in water/glycerol solutions. Fluids 4:3
Es-haghi H, Bouhendi H, Bagheri-Marandi G et al (2010) Cross-linked poly(acrylic acid) microgels from precipitation polymerization. Polym Plast Technol Eng 49:1257–1264
Es-haghi H, Bouhendi H, Marandi GB et al (2013) An investigation into novel multifunctional cross-linkers effect on microgel prepared by precipitation polymerization. React Funct Polym 73:524–530
Es-Haghi H, Bouhendi H, Marandi GB et al (2012) Rheological properties of microgel prepared with long-chain crosslinkers by a precipitation polymerization method. J Macromol Sci Part B 51:880–896
Nae HN, Reichert WW (1992) Rheological properties of lightly crosslinked carboxy copolymers in aqueous solutions. Rheol Acta 31:351–360
Pourjavadi A, Kohestanian M, Yaghoubi M (2019) Poly(glycidyl methacrylate)-coated magnetic graphene oxide as a highly efficient nanocarrier: Preparation, characterization, and targeted DOX delivery. New J Chem 43:18647–18656
Kohestanian M, Bouhendi H (2015) Novel cross-linking mechanism for producing PAA microgels synthesized by precipitation polymerization method. Colloid Polym Sci 293:1983–1995
Kohestanian M, Bouhendi H, Ghiass M (2017) Synthesis and characterization of PAA microgels using multifunctional epoxy cross-linkers with a new cross-linking mechanism via a precipitation polymerization method. J Polym Res 24:194
Zuo Y, He X, Li P et al (2019) Preparation and characterization of hydrophobically grafted starches by in situ solid phase polymerization. Polymers (Basel) 11:72
Otera J, Nishikido J (2009) Esterification, Method, Reaction, and Applications. Wiley-VCH
Wolfe MS, Scopazzi C (1989) Rheology of swellable microgel dispersions : influence of crosslink density. J Colloid Interface Sci 133:265–277
Pourjavadi A, Rahemipoor S, Kohestanian M (2020) Synthesis and characterization of multi stimuli-responsive block copolymer-silica hybrid nanocomposite with core-shell structure via RAFT polymerization. Compos Sci Technol 188:107951
Nigro V, Angelini R, Rosi B et al (2019) Study of network composition in interpenetrating polymer networks of poly(N isopropylacrylamide) microgels: the role of poly(acrylic acid). J Colloid Interface Sci 545:210–219
Kim JY, Song JY, Lee EJ, Park SK (2003) Rheological properties and microstructures of carbopol gel network system. Colloid Polym Sci 281:614–623
Islam MT, Rodríguez-Hornedo N, Ciotti S, Ackermann C (2004) Rheological characterization of topical carbomer gels neutralized to different pH. Pharm Res 21:1192–1199
Vanderhooft JL, Alcoutlabi M, Magda JJ, Prestwich GD (2009) Rheological properties of cross-linked hyaluronan-gelatin hydrogels for tissue engineering. Macromol Biosci 9:20–28
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kohestanian, M., Bouhendi, H., Keshavarzi, N. et al. Preparation of poly (acrylic acid) microgels by alcohol type cross-linkers and a comparison with other cross-linking methods. Polym. Bull. 79, 7775–7794 (2022). https://doi.org/10.1007/s00289-021-03878-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03878-5