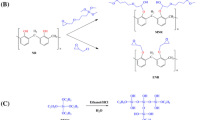
Abstract
Benzoxazine monomer containing chalcone moiety was prepared through Mannich condensation reaction. The monomer and monomer mixed with different ratios of graphene oxide were exposed to UV irradiation followed by thermal curing to produce pristine thermoset and nanocomposites, respectively. The monomers, pristine thermoset and nanocomposites were characterized by analytical techniques such as 1H-NMR, 13C-NMR, FTIR, SEM and XRD. The peak characteristic to oxazine ring disappeared in the pristine thermosets and nanocomposites confirming successful ring-opening polymerization. The (001) plane in GO was not observed in the nanocomposites revealing the dispersion of graphene layers in the polymer matrix, and the dispersion was confirmed by SEM examination. The thermal properties using TGA and DSC indicate that the presence of graphene oxide catalyzes the ring-opening of oxazine and decreases the curing temperature up to 80 °C compared with pure benzoxazine monomer. In addition, the nanocomposites exhibit higher thermal stability and electrical conductivity compared with the pristine thermoset.
Graphic abstract











Similar content being viewed by others
References
Zhang W, Zhan Y, Gao X, Li R, Zhu W, Xu H, Liu B, Fang X, Xu Y, Ding T (2018) EffecEt of oxygen functionalities of graphene oxide on polymerization and thermal properties of reactive benzoxazine nanocomposites. Macromol. Res 26:77–84. https://doi.org/10.1007/s13233-018-6009-0
Liu J, Agag T, Ishida H (2010) Main-chain benzoxazine oligomers: A new approach for resin transfer moldable neat benzoxazines for high performance applications. Polym J 51:5688–5694. https://doi.org/10.1016/j.polymer.2010.08.059
Wang J, Fang X, Wu M, He X, Liu W, Shen X (2011) Synthesis, curing kinetics and thermal properties of bisphenol-AP-based benzoxazine. Eur Polym J 47:2158–2168. https://doi.org/10.1016/j.eurpolymj.2011.08.005
Ke L, Hu D, Lu Y, Feng S, Xie Y, Xu W (2012) Copolymerization of maleimide-based benzoxazine with styrene and the curing kinetics of the resultant copolymer. Polym Degrad Stab 97:132–138. https://doi.org/10.1016/j.polymdegradstab.2011.11.011
Agag T, Jin L, Ishida H (2009) A new synthetic approach for difficult benzoxazines: Preparation and polymerization of 4, 4′-diaminodiphenyl sulfone-based benzoxazine monomer. Polym J 50:5940–5944. https://doi.org/10.1016/j.polymer.2009.06.038
Cao GP, Chen WJ, Liu XB (2008) Synthesis and thermal properties of the thermosetting resin based on cyano functionalized benzoxazine. Polym Degrad Stab 93:739–744. https://doi.org/10.1016/j.polymdegradstab.2007.10.002
Takeichi T, Agag T, Zeidam R (2001) Preparation and properties of polybenzoxazine/poly (imide-siloxane) alloys: In situ ring-opening polymerization of benzoxazine in the presence of soluble poly (imide-siloxane) s. J Polym Sci Part A Polym Chem 39:2633–2641. https://doi.org/10.1002/pola.1240
Kiskan B, Yagci Y (2007) Thermally curable benzoxazine monomer with a photodimerizable coumarin group . J Polym Sci Part A Polym Chem 45:1670–1676. https://doi.org/10.1002/pola.21934
Lin RC, Mohamed MG, Hsu KC, Wu JY, Jheng YR, Kuo SW (2016) Multivalent photo-crosslinkable coumarin-containing polybenzoxazines exhibiting enhanced thermal and hydrophobic surface properties. RSC Adv 6:10683–10696. https://doi.org/10.1039/C5RA27705A
Mohamed MG, Hsu KC, Kuo SW (2015) Bifunctional polybenzoxazine nanocomposites containing photo-crosslinkable coumarin units and pyrene unit capable of dispersing single-walled carbon nanotubes. Polym Chem 6:2423–2433. https://doi.org/10.1039/c5py00035a
Jin L, Agag T, Yagci Y, Ishida H (2011) Methacryloyl-functional benzoxazine: photopolymerization and thermally activated polymerization. Macromolecules 44:767–772. https://doi.org/10.1021/ma102351a
Koz B, Kiskan B, Yagci Y (2011) A novel benzoxazine monomer with methacrylate functionality and its thermally curable (co) polymers. Polym Bull 66:165–174. https://doi.org/10.1007/s00289-010-0261-6
Lin CH, Chien CK, Chen CH, Juang TY (2017) Photo-sensitive benzoxazine II: chalcone-containing benzoxazine and its photo and thermal-cured thermoset. RSC Adv 7:37844–37851
Agag T, Takeichi T (2003) Synthesis and characterization of novel benzoxazine monomers containing Allyl groups and their high performance thermosets. Macromolecules 36:6010–6016
Agag T, Takeichi T (2000) Polybenzoxazine–montmorillonite hybrid nanocomposites: synthesis and characterization. Polym J 41:7083–7090
Li W, Wei T, Gao Y, Xi K, Jia X (2012) Preparation of novel benzoxazine monomers containing ferrocene moiety and properties of polybenzoxazines. Polym J 53:1236–1244. https://doi.org/10.1016/j.polymer.2012.01.052
Frisch HL, Mark JE (1996) Nanocomposites prepared by threading polymer chains through zeolites, mesoporous silica, or silica nanotubes. Chem Mater 8:1735–1738
Agag T, Takeichi T (2000) Novel method for preparation of poly (benzoxazinone-imide). J Polym Sci Part A Polym Chem 38:1647–1655
Baqar M, Agag T, Ishida H, Qutubuddin S (2011) Poly (benzoxazine-co-urethane) s: a new concept for phenolic/urethane copolymers via one-pot method. Polym J 52:307–317. https://doi.org/10.1016/j.polymer.2010.11.052
Hariharan A, Prabunathan P, Subramanian SS, Kumarave M, Alagar M (2019) Blends of chalcone benzoxazine and bio-benzoxazines coated cotton fabrics for oil-water separation and bio-silica reinforced nanocomposites for low-k applications. J Polym Environ 28:598–613. https://doi.org/10.1007/s10924-019-01629-2
Hariharan1 A, Prabunathan1 P, Kumaravel A, Manoj M (2020) Bio-based polybenzoxazine composites for oil-water separation, sound absorption and corrosion resistance applications. Polym. Test 86–106443. http://www.elsevier.com/locate/polytest
Kumara S, Hariharanb A, Alagarb M, Dinakarana K (2020) Low-k and UV shielding polybenzoxazine nanocomposites synthesised from quinoline amine and bio-silica. Interfaces, Compos. https://doi.org/10.1080/09276440.2020.1833594
Latha G, Hariharan A, Prabunathan P, Alagar M (2020) Cardanol-imidazole based benzoxazine blends and bio-silica reinforced composites with enhanced surface, thermal and dielectric properties. J Polym Environ 28:918–933. https://doi.org/10.1007/s10924-019-01649-y
Potts JR, Dreyer DR, Bielawski CW, Ruoff RS (2011) Graphene-based Polymer nanocomposites . Polym J 52:5–25. https://doi.org/10.1016/j.polymer.2010.11.042
Stankovich S, Dikin DA, Dommett GH, Kohlhaas KM, Zimney EJ, Stach EA, Piner RD, Nguyen ST, Ruoff RS (2006) Graphene-based composite materials. Nature 448:282–286. https://doi.org/10.1038/nature04969
Alhassan SM, Qutubuddin S, Schiraldi DA, Agag T, Ishida H (2013) Preparation and thermal properties of graphene oxide/main chain benzoxazine. Eur Polym J 49:3825–3833. https://doi.org/10.1016/j.eurpolymj.2013.09.005
Zeng M, Wang J, Li R, Liu J, Chen W, Xu Q, Gu Y (2013) The curing behavior and thermal property of graphene oxide/benzoxazine nanocomposites. Polym J 54:3107–3116. https://doi.org/10.1016/j.polymer.2013.03.069
Alhwaige AA, Alhassan SM, Katsiotis MS, Ishida H, Qutubuddin S (2015) Interactions, morphology and thermal stability of graphene-oxide reinforced polymer aerogels derived from star-like telechelic aldehyde-terminal benzoxazine resin. RSC Adv 5:92719–92731. https://doi.org/10.1039/c5ra16188f
Jo MJ, Choi H, Kim GH, Yu WR, Park M, Kim Y, Park JK, Youk JH (2018) Preparation of epoxy shape memory polymers for deployable space structures using flexible diamines. Fibers Polym 19:1799–1805. https://doi.org/10.1007/s12221-018-8549-5
Meng F, Ishida H, Liu X (2014) Introduction of benzoxazine onto the graphene oxide surface by click chemistry and the properties of graphene oxide reinforced polybenzoxazine nanohybrids. RSC Adva 4:9471–9475. https://doi.org/10.1039/c3ra47345g
Lu Y, Zhang S, Geng Z, Zhu K, Zhang M, Na R, Wang G (2017) Hybrid formation of graphene oxide–POSS and their effect on the dielectric properties of poly (aryl ether ketone) composites. New J Chem 41:3089–3096. https://doi.org/10.1039/C6NJ03802F
Liu X, Yang J, Zhao W, Wang Y, Li Z, Lin Z (2016) A simple route to reduced graphene oxide-draped nanocomposites with markedly enhanced visible-light photocatalytic performance. Small 12:4077–4085. https://doi.org/10.1002/smll.201601110
Kim H, Abdala AA, Macosko CW (2010) Graphene/polymer nanocomposites. Macromolecules 43:6515–6530. https://doi.org/10.1021/ma100572e
Biru I, Damian CM, Gârea SA, Iovu H (2016) Benzoxazine-functionalized graphene oxide for synthesis of new nanocomposites. Eur Polym J 283:244–255. https://doi.org/10.1016/j.eurpolymj.2016.08.024
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, Alemany LB, Lu W, Tour JM (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814
Zaaba NI, Foo KL, Hashim U, Tan SJ, Liu WW, Voon CH (2017) Synthesis of graphene oxide using modified hummers method: solvent influence. Procedia Eng 184:469–477. https://doi.org/10.1016/j.proeng.2017.04.118
Rathnayake RMNM, Wijayasinghe HWMAC, Pitawala HMTGA, Yoshimura M, Huang HH (2017) Synthesis of graphene oxide and reduced graphene oxide by needle platy natural vein graphite . Appl Surf Sci 393:309–315. https://doi.org/10.1016/j.apsusc.2016.10.008
Rehab A (1998) New photosensitive polymers as negative photoresist materials. Eur Polym J 12:1845–1855
Fleming I, Williams DH (1966) Spectroscopic methods in organic chemistry. McGraw- Hill New York.
Muthukaruppan A, Arumugam H, Krishnan S, Kannan K, Chavali M (2018) A low cure thermo active polymerization of chalcone based benzoxazine and cross linkable olefin blends. J Polym Res 25:163. https://doi.org/10.1007/s10965-018-1556-9
Zhou Y, Bao Q, Tang LAL, Zhong Y, Loh KP (2009) Hydrothermal dehydration for the “green” reduction of exfoliated graphene oxide to graphene and demonstration of tunable optical limiting properties. Chem Mater 21:2950–2956. https://doi.org/10.1021/cm9006603
Alateyah AI (2018) Thermal properties and morphology of polypropylene based on exfoliated graphite nanoplatelets/nanomagnesium oxide. Sag Open Eng. 8:432–439
Lin CH, Chen ZJ, Chen CH, Wang MW, Juang TY (2017) Synthesis of a bisbenzylideneacetone-containing benzoxazine and its photo-and thermally cured thermoset. ACS Omega 2:3432–3440. https://doi.org/10.1021/acsomega.7b00573
Tie W, Zhong Z, Li L, Zhang A, Shen F, Lee MH, Li XD (2012) Synthesis and characterization of novel photosensitive polysulfones with photocrosslinkable side pendants. Eur Polym J 48:2070–2075. https://doi.org/10.1016/j.eurpolymj.2012.09.002
Tian X, Itkis ME, Bekyarova EB, Haddon RC (2013) anisotropic thermal and electrical properties of thin thermal interface layers of graphite nanoplatelet-based composite. Sci Rep 3:1. https://doi.org/10.1038/srep01710
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salahuddin, N., Rehab, A., El-Deeb, I.Y. et al. Effect of graphene oxide on photo- and thermal curing of chalcone–based benzoxazine resins. Polym. Bull. 79, 3175–3191 (2022). https://doi.org/10.1007/s00289-021-03590-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03590-4