Abstract
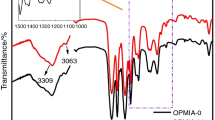
An energetic triblock copolymer PPG–PGN–PPG (Mn = 1886 g mol−1) was synthesized for the first time by cationic ring-opening polymerization of propylene oxide with low molecular weight poly glycidyl nitrate (PGN) (Mn = 1061 g mol−1) as a macroinitiator, in the presence of boron trifluoride etherate (BF3·OEt2) as the catalyst. The product obtained in high yield was characterized by FTIR, gel permeation chromatography and 1H and 13C NMR spectroscopy. The thermal properties of the triblock copolymer were characterized by differential scanning calorimeter (DSC). The result approved that the glass transition temperature of the triblock copolymer (Tg = − 58 °C) is lower than PGN (Tg = − 35 °C); also, it was more stable than that of PGN. The effect of heating rates (10, 20, 30 and 40 °C min−1) on the decomposition of the copolymer was evaluated. The decomposition temperature of this compound increased as the heating rate increased. The kinetic parameters such as activation energy and frequency factor for the thermal decomposition of the triblock copolymer were obtained from the DSC and DTG data by non-isothermal methods proposed by the ASTM E696, Flynn–Wall–Ozawa (FWO) and Kissinger methods. The values by the FWO method are in good agreement with ASTM and Kissinger methods.














Similar content being viewed by others
References
Mohan YM, Mani Y, Raju KM (2006) Synthesis of azido polymers as potential energetic propellant binders. Des Monomers Polym 9(3):201–236
Chaturvedi S, Dave PN (2015) Solid propellants: AP/HTPB composite propellants. Arab J Chem. https://doi.org/10.1016/j.arabjc.2014.12.033
Agrawal JP (2010) High energy materials: propellants, explosives and pyrotechnics. Wiley, Hoboken
Jain S (2002) Solid propellant binders. J Sci Ind Res 61:899–911
COUTURIER R (1993) Advanced energetic binder propellants. In: Solid Rocket Propulsion Technology. Elsevier, Amsterdam, pp 477–524
Provatas A (2000) Energetic polymers and plasticisers for explosive formulations—a review of recent advances. Defence Science and Technology Organisation Melbourne (Australia)
Bayat Y, Bayat MH (2018) Study of thermal analysis and kinetic decomposition of polybutadiene acrylonitrile acrylic acid (PBAN). J Therm Anal Calorim 134(2):1091–1100
Diaz E, Brousseau P, Ampleman G, Prud’homme RE (2003) Heats of combustion and formation of new energetic thermoplastic elastomers based on GAP, PolyNIMMO and PolyGLYN. Propellants Explos Pyrotech 28(3):101–106
Agrawal JP (1998) Recent trends in high-energy materials. Prog Energy Combust Sci 24(1):1–30
Selim K, Özkar S, Yilmaz L (2000) Thermal characterization of glycidyl azide polymer (GAP) and GAP-based binders for composite propellants. J Appl Polym Sci 77(3):538–546
Shee SK, Reddy ST, Athar J, Sikder AK, Talawar M, Banerjee S, Khan MAS (2015) Probing the compatibility of energetic binder poly-glycidyl nitrate with energetic plasticizers: thermal, rheological and DFT studies. RSC Adv 5(123):101297–101308
Kishore K, Sridhara K (1999) Solid propellant chemistry: condensed phase behaviour of ammonium perchlorate-based solid propellants. Defence Research & Development Organisation, Ministry of Defence
Provatas A (2003) Energetic plasticizer migration studies. Energ Mater 21(4):237–245
Bhowmik D, Sadavarte VS, Pande SM, Saraswat BS (2015) An energetic binder for the formulation of advanced solid rocket propellants. Cent Eur J Energ Mater 12(1):145–148
Talawar M, Sivabalan R, Mukundan T, Muthurajan H, Sikder A, Gandhe B, Rao AS (2009) Environmentally compatible next generation green energetic materials (GEMs). J Hazard Mater 161(2–3):589–607
Desai H, Cunliffe A, Lewis T, Millar R, Paul N, Stewart M, Amass A (1996) Synthesis of narrow molecular weight α, ω-hydroxy telechelic poly(glycidyl nitrate) and estimation of theoretical heat of explosion. Polymer 37(15):3471–3476
Colclough ME, Desai H, Millar RW, Paul NC, Stewart MJ, Golding P (1994) Energetic polymers as binders in composite propellants and explosives. Polym Adv Technol 5(9):554–560
Dong Q, Li H, Liu X, Huang C (2018) Thermal and rheological properties of PGN, PNIMMO and P (GN/NIMMO) synthesized via mesylate precursors. Propellants Explos Pyrotech 43(3):294–299
Ang HG, Pisharath S (2012) Energetic polymers: binders and plasticizers for enhancing performance. Wiley, Hoboken
Abrishami F, Zohari N, Zeynali V (2019) Synthesis and kinetic study on the thermal degradation of triblock copolymer of polycaprolactone–poly(glycidyl nitrate)–polycaprolactone (PCL–PGN–PCL) as an energetic binder. Polym Adv Technol 30(3):640–647
Ghorbani YBM, Mohammadi MM, Jafari S (2014) Synthesis and characterization of PGN–PTHF–PGN as a new triblock copolymer. In: 11th International seminar on polymer science and technology. https://www.civilica.com/Paper-ISPST11-ISPST11_272.html
Abrishami F, Zohari N, Zeynali V (2017) Synthesis and characterization of poly(glycidyl nitrate-block-caprolactone-block-glycidyl nitrate)(PGN-PCL-PGN) tri-block copolymer as a novel energetic binder. Propellants Explos Pyrotech 42(9):1032–1036
Ghorbani M, Bayat Y (2015) Synthesis and characterization of hydroxyl-terminated triblock copolymer of poly(glycidyl nitrate-block-butadiene-block-glycidyl nitrate) as potential energetic binder. Polym Sci Ser B 57(6):654–658
Sanderson AJ, Martins LJ, Dewey MA (2005) Process for making stable cured poly(glycidyl nitrate) and energetic compositions comprising same. Google Patents
Herzberger J, Niederer K, Pohlit H, Seiwert J, Worm M, Wurm FR, Frey H (2015) Polymerization of ethylene oxide, propylene oxide, and other alkylene oxides: synthesis, novel polymer architectures, and bioconjugation. Chem Rev 116(4):2170–2243
Ertem SP, Yilgor E, Kosak C, Wilkes GL, Zhang M, Yilgor I (2012) Effect of soft segment molecular weight on tensile properties of poly(propylene oxide) based polyurethaneureas. Polymer 53(21):4614–4622
Wang Q, Wang L, Zhang X, Mi Z (2009) Thermal stability and kinetic of decomposition of nitrated HTPB. J Hazard Mater 172(2–3):1659–1664
Martin J, Cadenato A, Salla J (1997) Comparative studies on the non-isothermal DSC curing kinetics of an unsaturated polyester resin using free radicals and empirical models. Thermochim Acta 306(1–2):115–126
Hassan M, Shehata A (2002) Studies on some acrylamido polymers and copolymer as stabilizers for nitrocellulose. J Appl Polym Sci 85(14):2808–2819
Lu Y-C, Kuo KK (1996) Thermal decomposition study of hydroxyl-terminated polybutadiene (HTPB) solid fuel. Thermochim Acta 275(2):181–191
Yi J-h, Zhao F-q, Xu S-y, Zhang L-y, Gao H-x (2009) Effects of pressure and TEGDN content on decomposition reaction mechanism and kinetics of DB gun propellant containing the mixed ester of TEGDN and NG. J Hazard Mater 165(1–3):853–859
Ma H, Yan B, Li Z, Guan Y, Song J, Xu K, Hu R (2009) Preparation, non-isothermal decomposition kinetics, heat capacity and adiabatic time-to-explosion of NTO· DNAZ. J Hazard Mater 169(1–3):1068–1073
Pourmortazavi S, Hosseini S, Rahimi-Nasrabadi M, Hajimirsadeghi S, Momenian H (2009) Effect of nitrate content on thermal decomposition of nitrocellulose. J Hazard Mater 162(2–3):1141–1144
Keshavarz MH (2009) Simple method for prediction of activation energies of the thermal decomposition of nitramines. J Hazard Mater 162(2–3):1557–1562
Rocco JAFF, Lima JES, Frutuoso AG, Iha K, Ionashiro M, Matos JdR, Suárez-Iha MEV (2004) Thermal degradation of a composite solid propellant examined by DSC. J Therm Anal Calorim 75(2):551–557
Roduit B, Xia L, Folly P, Berger B, Mathieu J, Sarbach A, Andres H, Ramin M, Vogelsanger B, Spitzer D (2008) The simulation of the thermal behavior of energetic materials based on DSC and HFC signals. J Therm Anal Calorim 93(1):143–152
Zhang W, Ren H, Sun Y, Yan S, Jiao Q (2018) Effects of ester-terminated glycidyl azide polymer on the thermal stability and decomposition of GAP by TG-DSC-MS-FTIR and VST. J Therm Anal Calorim 132(3):1883–1892
Guo M, Ma Z, He L, He W, Wang Y (2017) Effect of varied proportion of GAP-ETPE/NC as binder on thermal decomposition behaviors, stability and mechanical properties of nitramine propellants. J Therm Anal Calorim 130(2):909–918
Pisharath S, Ang HG (2007) Synthesis and thermal decomposition of GAP–Poly (BAMO) copolymer. Polym Degrad Stab 92(7):1365–1377
Zhang J, Xue B, Rao G, Chen L, Chen W (2018) Thermal decomposition characteristic and kinetics of DINA. J Therm Anal Calorim 133(1):727–735
Gołofit T, Zyśk K (2015) Thermal decomposition properties and compatibility of CL-20 with binders HTPB, PBAN, GAP and polyNIMMO. J Therm Anal Calorim 119(3):1931–1939
Lua AC, Su J (2006) Isothermal and non-isothermal pyrolysis kinetics of Kapton® polyimide. Polym Degrad Stab 91(1):144–153
Sivalingam G, De P, Karthik R, Madras G (2004) Thermal degradation kinetics of vinyl polyperoxide copolymers. Polym Degrad Stab 84(1):173–179
Morancho J, Salla J, Ramis X, Cadenato A (2004) Comparative study of the degradation kinetics of three powder thermoset coatings. Thermochim Acta 419(1–2):181–187
Tuffi R, D’Abramo S, Cafiero L, Trinca E, Ciprioti SV (2018) Thermal behavior and pyrolytic degradation kinetics of polymeric mixtures from waste packaging plastics. Express Polym Lett 12(1):82–99
Ries A, Canedo EL, Souto CR, Wellen RM (2016) Non-isothermal cold crystallization kinetics of poly(3-hydoxybutyrate) filled with zinc oxide. Thermochim Acta 637:74–81
Singh A, Sharma TC, Kumar M, Narang JK, Kishore P, Srivastava A (2017) Thermal decomposition and kinetics of plastic bonded explosives based on mixture of HMX and TATB with polymer matrices. Def Technol 13(1):22–32
Li H, Pan R, Wang W, Zhang L (2014) Thermal decomposition and kinetics studies on poly (BDFAO/THF), poly (DFAMO/THF), and poly (BDFAO/DFAMO/THF). J Therm Anal Calorim 118(1):189–196
Howell B, Sastry B (1999) Comparison of the activation energies for the thermal decomposition of copper and nickel compounds obtained by various thermogravimetric methods. Thermochim Acta 340:311–314
Wang H, Tao X, Newton E (2004) Thermal degradation kinetics and lifetime prediction of a luminescent conducting polymer. Polym Int 53(1):20–26
Wan C, Tian G, Cui N, Zhang Y, Zhang Y (2004) Processing thermal stability and degradation kinetics of poly(vinyl chloride)/montmorillonite composites. J Appl Polym Sci 92(3):1521–1526
Satoh K, Kamigaito M, Sawamoto M (2000) Direct living cationic polymerization of p-hydroxystyrene with boron trifluoride etherate in the presence of water. Macromolecules 33(15):5405–5410
Uyar T, Hacaloğlu J (2002) Thermal degradation of poly(propylene oxide) and polyepichlorohydrin by direct pyrolysis mass spectrometry. J Anal Appl Pyrol 64(2):379–393
Zhang Z, Wang G, Luo N, Huang M, Jin M, Luo Y (2014) Thermal decomposition of energetic thermoplastic elastomers of poly(glycidyl nitrate). J Appl Polym Sci. https://doi.org/10.1002/app.40965
Song LX, Guo XQ, Du FY, Bai L (2010) Thermal degradation comparison of polypropylene glycol and its complex with β-cyclodextrin. Polym Degrad Stab 95(4):508–515
Salla J, Morancho J, Cadenato A, Ramis X (2003) Non-isothermal degradation of a thermoset powder coating in inert and oxidant atmospheres. J Therm Anal Calorim 72(2):719–728
Li L, Guan C, Zhang A, Chen D, Qing Z (2004) Thermal stabilities and the thermal degradation kinetics of polyimides. Polym Degrad Stab 84(3):369–373
ASTM E (2005) 698-05 Standard test method for Arrhenius kinetic constants for thermally unstable materials using differential scanning calorimetry and the Flynn. Wall/Ozawa Method
Sunitha M, Nair CR, Krishnan K, Ninan K (2001) Kinetics of Alder-ene reaction of Tris (2-allylphenoxy) triphenoxycyclotriphosphazene and bismaleimides—a DSC study. Thermochim Acta 374(2):159–169
Sovizi M, Hajimirsadeghi S, Naderizadeh B (2009) Effect of particle size on thermal decomposition of nitrocellulose. J Hazard Mater 168(2–3):1134–1139
Tompa AS, Boswell RF (2000) Thermal stability of a plastic bonded explosive. Thermochim Acta 357:169–175
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khanlari, T., Bayat, Y. & Bayat, M. Synthesis, thermal stability and kinetic decomposition of triblock copolymer polypropylene glycol–poly glycidyl nitrate–polypropylene glycol (PPG–PGN–PPG). Polym. Bull. 77, 5859–5878 (2020). https://doi.org/10.1007/s00289-019-03051-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-03051-z