Abstract
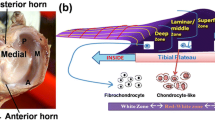
The structural complexity of human knee meniscus warrants a suitable 3D microenvironment for its successful regeneration. For this, 3D porous poly-ε-caprolactone (PCL) scaffold structures were prepared using freeze drying method (lyophilization). PCL was dissolved in different weight percentages (10, 15, 20% w/v, coded as P10, P15 and P20, respectively) in acetone and lyophilized to develop 3D porous structures. The scaffolds were studied for their morphological, mechanical and functional properties. Compression tests were performed to understand their compressive properties and structural integrity. Results indicate that P10 is most suitable considering the highest porosity (above 85%), uniform pore distribution, better resiliency and less than 40% compression at a load of 30 kg/cm2 for 1 min. Further, the effect of a unique combination of biomolecule (UCM) functionalization in 3D PCL scaffold (P10) on human meniscal cell attachment, growth and proliferation was studied in detail. The developed scaffolds were also characterized for dynamic mechanical properties and compared with native human meniscal tissue properties. The primary human meniscus cell attachment, growth and proliferation on to the scaffolds were studied and analyzed using SEM and Hoechst staining experiments. Extracellular matrix secretion (glycosaminoglycans and collagen) in the cell culture medium was estimated. UCM-functionalized PCL 3D porous scaffold, P10 showed better cell attachment and proliferation indicating its potential for meniscus tissue engineering applications.








Similar content being viewed by others
References
Amin NH, Hussain W, Ryan J, Morrison S, Miniaci A, Jones MH (2017) Changes within clinical practice after a randomized controlled trial of knee arthroscopy for osteoarthritis. Orthopaedic Soc Sports Med 5:2325967117698439
Weinand C, Peretti GM, Adams SB, Randolph MA, Savvidis E, Gill TJ (2006) Healing potential of transplanted allogeneic chondrocytes of three different sources in lesions of the avascular zone of the meniscus: a pilot study. Arch Orthop Trauma Surg 126:599–605
Moran CJ, Busilacchi A, Lee CA, Athanasiou KA, Verdonk PC (2015) Biological augmentation and tissue engineering approaches in meniscus surgery. Arthroscopy 31:944–955
Das RK, Zouani OF (2014) A review of the effects of the cell environment physicochemical nanoarchitecture on stem cell commitment. Biomaterials 35:5278–5293
Costa JB, Oliveira JM, Reis RL (2017) Biomaterials in meniscus tissue engineering. In: Oliveira J, Reis R (eds) Regenerative strategies for the treatment of knee joint disabilities. Studies in mechanobiology, tissue engineering and biomaterials, vol 21. Springer, Cham. https://doi.org/10.1007/978-3-319-44785-8
Baker BM, Nathan AS, Huffman GR, Mauck RL (2009) Tissue engineering with meniscus cells derived from surgical debris. Osteoarthr Cartil 17:336–345
Lee CH, Rodeo SA, Fortier LA, Lu C, Erisken C, Mao JJ (2014) Protein-releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep. Sci Transl Med 6:266ra171
Peretti GM, Gill TJ, Xu JW, Randolph MA, Morse KR, Zaleske DJ (2004) Cell-based therapy for meniscal repair. Am J Sports Med 32:146–158
Kang SW, Sun-Mi S, Jae-Sun L, Eung-Seok L, Kwon-Yong L, Sang-Guk P, Jung-Ho P, Byung-Soo K (2006) Regeneration of whole meniscus using meniscal cells and polymer scaffolds in a rabbit total meniscectomy model. J Biomed Mater Res A 77:659–671
Moran CJ, Busilacchi A, Lee CA, Athanasiou KA, Verdonk PC (2015) Biological augmentation and tissue engineering approaches in meniscus surgery. Arthroscopy 31:944–955
Pillai MM, Elakkiya V, Gopinathan J, Sabarinath C, Shanthakumari S, Sahanand KS, Rai BD, Bhattacharyya A, Selvakumar R (2016) A combination of biomolecules enhances expression of E-cadherin and peroxisome proliferator-activated receptor gene leading to increased cell proliferation in primary human meniscal cells: an in vitro study. Cytotechnology 68:1747–1761
Gopinathan J, Mano S, Elakkiya V, Pillai MM, Sahanand KS, Rai BD, Selvakumar R, Bhattacharyya A (2015) Biomolecule incorporated poly-ε-caprolactone nanofibrous scaffolds for enhanced human meniscal cell attachment and proliferation. RSC Adv 5:73552–73561
Gopinathan J, Pillai MM, Sahanand KS, Rai BD, Selvakumar R, Bhattacharyya A (2017) Synergistic effect of electrical conductivity and biomolecules on human meniscal cell attachment, growth and proliferation in poly-ε-caprolactone nanocomposite scaffolds. Biomed Mater 12:065001
Pillai MM, Gopinathan J, Senthil Kumar R, Sathish Kumar G, Shanthakumari S, Sahanand KS, Bhattacharyya A, Selvakumar R (2018) Tissue engineering of human knee meniscus using functionalized and reinforced Silk-PVA composite 3D scaffolds: Understanding the in vitro and in vivo behaviour. J Biomed Mater Res Part A. https://doi.org/10.1002/jbm.a.36372
Nazarov R, Jin HJ, Kaplan DL (2004) Porous 3-D scaffolds from regenerated silk fibroin. Biomacromol 5:718–726
Pillai MM, Akshaya TR, Elakkiya V, Gopinathan J, Sahanand KS, Rai BD, Bhattacharyya A, Selvakumar R (2015) Egg shell membrane–a potential natural scaffold for human meniscal tissue engineering: an in vitro study. RSC Adv 5:76019–76025
Pillai MM, Gopinathan J, Indumathi B, Manjoosha YR, Sahanand KS, Rai BD, Selvakumar R, Bhattacharyya A (2016) Silk–PVA hybrid nanofibrous scaffolds for enhanced primary human meniscal cell proliferation. J Membr Biol 249:813–822
Nguyen TH, Bao TQ, Park I, Lee BT (2013) A novel fibrous scaffold composed of electrospun porous poly (ε-caprolactone) fibers for bone tissue engineering. J Biomater Appl 28:514–528
Nakamatsu J, Torres FG, Troncoso OP, Min-Lin Y, Boccaccini AR (2006) Processing and characterization of porous structures from chitosan and starch for tissue engineering scaffolds. Biomacromol 7(12):3345–3355
Panadero JA, Vikingsson L, Gomez Ribelles JL, Lanceros-Mendez S, Sencadas V (2015) Inn vitro mechanical fatigue behavior of poly-ε-caprolactone macroporous scaffolds for cartilage tissue engineering: influence of pore filling by a poly (vinyl alcohol) gel. J Biomed Mater Res B Appl Biomater 103(5):1037–1043
Athanasiou KA, Sanchez-Adams J (2009) Engineering the knee meniscus. Synth Lect Tissue Eng 1:1–97
Moore EE, Bendele AM, Thompson DL, Littau A, Waggie KS, Reardon B, Ellsworth JL (2005) Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthritis Cartil 13(7):623–631
Guo B, Lei B, Li P, Ma PX (2015) Functionalized scaffolds to enhance tissue regeneration. Regen Biomater 2(1):47–57
Rongen JJ, van Tienen TG, van Bochove B, Grijpma DW, Buma P (2014) Biomaterials in search of a meniscus substitute. Biomaterials 35:3527–3540
Bassi AK, Gough JE, Zakikhani M, Downes S (2011) The chemical and physical properties of poly (ε-caprolactone) scaffolds functionalised with poly (vinyl phosphonic acid-co-acrylic acid). J Tissue Eng 2:615328. https://doi.org/10.4061/2011/615328
Polini A, Pisignano D, Parodi M, Quarto R, Scaglione S (2011) Osteoinduction of human mesenchymal stem cells by bioactive composite scaffolds without supplemental osteogenic growth factors. PLoS ONE 6:e26211
Ibrahim M, Alaam M, El-Haes H, Jalbout AF, Leon AD (2006) Analysis of the structure and vibrational spectra of glucose and fructose. Eclet Quim 31:15–21
Wang DA, Varghese S, Sharma B, Strehin I, Fermanian S, Gorham J, Fairbrother DH, Cascio B, Elisseeff JH (2007) Multifunctional chondroitin sulphate for cartilage tissue–biomaterial integration. Nat Mater 6:385–392
Iyanar M, Prakash JT, Muthamizhchelvan C, Ekadevasena S, Gnanaraj JM, Dhavud SS (2015) Growth and characterization of l-proline doped adp crystals for optoelectronic applications. Mater Sci Res India 12:22–27
Chia HN, Hull ML (2008) Compressive moduli of the human medial meniscus in the axial and radial directions at equilibrium and at a physiological strain rate. J Orthop Res 26:951–956
Jiang T, Khan Y, Nair LS, Abdel-Fattah WI, Laurencin CT (2010) Functionalization of chitosan/poly (lactic acid-glycolic acid) sintered microsphere scaffolds via surface heparinization for bone tissue engineering. J Biomed Mater Res A 93:1193–1208
Acknowledgements
Funding was provided by PSG Management.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest.
Statement of human and animal rights
The authors declare that they have no ethical issues of human and animal right.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Janarthanan, G., Pillai, M.M., Kulasekaran, S.S. et al. Engineered knee meniscus construct: understanding the structure and impact of functionalization in 3D environment. Polym. Bull. 77, 2611–2629 (2020). https://doi.org/10.1007/s00289-019-02874-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-02874-0