Abstract
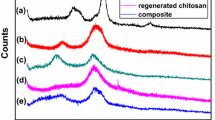
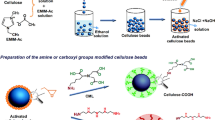
The use of nontoxic, biodegradable and biocompatible biopolymers such as chitosan and cellulose is of considerable importance particularly in uptake of metal ions from the contaminated water systems. Current study was aimed to develop a chitosan-based biosorbent in combination with cellulose to form composite adsorbent beads. The developed composite beads were chemo-physically characterized using advanced analytical techniques of scanning electron microscope, Fourier transform infrared, x-ray diffraction and thermogravimetric analysis. The point of zero charge (PZC) of the developed beads was determined using the methods of salt addition, electrokinetic potential and the mass atitration. The average value of PZC of composite beads was found to be 7.26 using the said methods. On incorporating cellulose into chitosan, the thermal properties of the composite beads were considerably enhanced. The adsorption capacity of chitosan–cellulose beads was found to be 99.8, 79.98 and 99.10 mg/g for Ni(II), Cu(II) and Cr(III), respectively. The adsorption extent showed that the developed composite beads could be employed as adsorbent for wastewater treatment.










Similar content being viewed by others
References
Martínez-Ruvalcaba A, Chornet E, Rodrigue D (2007) Viscoelastic properties of dispersed chitosan/xanthan hydrogels. Carbohydr Polym 67:586–595
Liu B, Wang D, Yu G, Meng X (2013) Adsorption of heavy metal ions, dyes and proteins by chitosan composites and derivatives—a review. J Ocean Univ China 12:500–508
Yadu Nath VK, Raghvendra Kumar M, Aswathy V, Parvathy P, Sunija S, Neelakandan MS, Nitheesha S, Vishnu KA (2017) Chitosan as promising materials for biomedical application: review. Res Dev Mater Sci 2:2576–8840
Kumar MNR (2000) A review of chitin and chitosan applications. React Funct Polym 46:1–27
Wang J, Chen C (2014) Chitosan-based biosorbents: modification and application for biosorption of heavy metals and radionuclides. Biores Technol 160:129–141
Ngah WW, Teong L, Hanafiah M (2011) Adsorption of dyes and heavy metal ions by chitosan composites: a review. Carbohydr Polym 83:1446–1456
Hokkanen S, Bhatnagar A, Sillanpää M (2016) A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res 91:156–173
Watermann T, Sebastiani D (2017) Liquid water confined in cellulose with variable interfacial hydrophilicity. Zeitschrift für Physikalische Chemie. https://doi.org/10.1515/zpch-2017-1011
Khan A, Khan RA, Salmieri S, Le Tien C, Riedl B, Bouchard J, Chauve G, Tan V, Kamal MR, Lacroix M (2012) Mechanical and barrier properties of nanocrystalline cellulose reinforced chitosan based nanocomposite films. Carbohydr Polym 90:1601–1608
Peng S, Meng H, Ouyang Y, Chang J (2014) Nanoporous magnetic cellulose–chitosan composite microspheres: preparation, characterization, and application for Cu(II) adsorption. Ind Eng Chem Res 53:2106–2113
Lin W-C, Lien C-C, Yeh H-J, Yu C-M, S-h Hsu (2013) Bacterial cellulose and bacterial cellulose–chitosan membranes for wound dressing applications. Carbohydr Polym 94:603–611
Zhou Y, Fu S, Zhang L, Zhan H, Levit MV (2014) Use of carboxylated cellulose nanofibrils-filled magnetic chitosan hydrogel beads as adsorbents for Pb(II). Carbohydr Polym 101:75–82
Mustafa S, Tasleem S, Naeem A (2004) Surface charge properties of Fe2O3 in aqueous and alcoholic mixed solvents. J Colloid Interface Sci 275:523–529
Fiol N, Villaescusa I (2009) Determination of sorbent point zero charge: usefulness in sorption studies. Environ Chem Lett 7:79–84
Mahmood T, Saddique MT, Naeem A, Westerhoff P, Mustafa S, Alum A (2011) Comparison of different methods for the point of zero charge determination of NiO. Ind Eng Chem Res 50:10017–10023
Li N, Bai R (2005) Copper adsorption on chitosan–cellulose hydrogel beads: behaviors and mechanisms. Sep Purif Technol 42:237–247
Munim SA, Saddique MT, Raza ZA, Majeed MI (2018) Preparation and physico-chemical characterization of β-cyclodextrin incorporated chitosan biosorbent beads with potential environmental applications. Mater Res Express. https://doi.org/10.1088/2053-1591/aac707
Zhao F, Repo E, Yin D, Sillanpää ME (2013) Adsorption of Cd(II) and Pb(II) by a novel EGTA-modified chitosan material: kinetics and isotherms. J Colloid Interface Sci 409:174–182
Fernandes Queiroz M, Melo KRT, Sabry DA, Sassaki GL, Rocha HAO (2014) Does the use of chitosan contribute to oxalate kidney stone formation? Mar Drugs 13:141–158
Nair V, Panigrahy A, Vinu R (2014) Development of novel chitosan–lignin composites for adsorption of dyes and metal ions from wastewater. Chem Eng J 254:491–502
Riva GH, García-Estrada J, Vega B, López-Dellamary F, Hérnandez ME, Silva JA (2015) Cellulose-fundamental aspects and current trends. Cellulose-chitosan nanocomposites-evaluation of physical, mechanical and biological properties. InTech, Rijeka
de Mesquita JP, Donnici CL, Pereira FV (2010) Biobased nanocomposites from layer-by-layer assembly of cellulose nanowhiskers with chitosan. Biomacromolecules 11:473–480
Mathew AP, Laborie M-PG, Oksman K (2009) Cross-linked chitosan/chitin crystal nanocomposites with improved permeation selectivity and pH stability. Biomacromolecules 10:1627–1632
Tian F, Liu Y, Hu K, Zhao B (2004) Study of the depolymerization behavior of chitosan by hydrogen peroxide. Carbohydr Polym 57:31–37
Lu X, Shen X (2011) Solubility of bacteria cellulose in zinc chloride aqueous solutions. Carbohydr Polym 86:239–244
Bagchi S, Kumar KJ (2016) Studies on water soluble polysaccharides from Pithecellobium dulce (Roxb.) Benth. seeds. Carbohydr Polym 138:215–221
George J, Ramana K, Bawa A (2011) Bacterial cellulose nanocrystals exhibiting high thermal stability and their polymer nanocomposites. Int J Biol Macromol 48:50–57
Lim B, Poh C, Voon C, Salmah H (2015) Rheological and thermal study of chitosan filled thermoplastic elastomer composites. In: Applied mechanics and materials. Trans Tech Publications, pp 34–38
Zhu B, Xia P, Ho W, Yu J (2015) Isoelectric point and adsorption activity of porous g-C3N4. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2015.03.086
Hasan S, Ghosh TK, Viswanath DS, Boddu VM (2008) Dispersion of chitosan on perlite for enhancement of copper(II) adsorption capacity. J Hazard Mater 152:826–837
Tirtom VN, Dinçer A, Becerik S, Aydemir T, Çelik A (2012) Comparative adsorption of Ni(II) and Cd(II) ions on epichlorohydrin crosslinked chitosan–clay composite beads in aqueous solution. Chem Eng J 197:379–386
Ngah WW, Fatinathan S (2008) Adsorption of Cu(II) ions in aqueous solution using chitosan beads, chitosan–GLA beads and chitosan–alginate beads. Chem Eng J 143:62–72
Acknowledgements
The authors recognize the financial Grant (NRPU-3153) of Higher Education Commission, Islamabad, for this study.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Munim, S.A., Saddique, M.T., Raza, Z.A. et al. Fabrication of cellulose-mediated chitosan adsorbent beads and their surface chemical characterization. Polym. Bull. 77, 183–196 (2020). https://doi.org/10.1007/s00289-019-02711-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-02711-4