Abstract
In this study, the ZnFe2O4@SiO2@Tragacanth gum magnetic nanocomposite as a novel adsorbent was synthesized and was confirmed by FTIR, XRD, TEM, TGA and VSM methods. Then, the prepared nanocomposite has been tested for the removal of methylene blue dye from aqueous solution for adsorption process, and the effect of various parameters including pH (2–12), adsorbent dosage (0.2–2 g/L), temperature (15–45 °C), initial dye concentration (10–60 mg/L) and contact time (5–120 min), also its isotherms and kinetics has been studied. The results showed that the optimum pH, contact time, initial dye concentration, adsorbent dosage and maximum adsorption capacity were 8, 60 min, 60 mg/L, 0.2 g/L and 109.37 mg/g, respectively. Furthermore, the experimental data determined that the adsorption of methylene blue dye interpreted the Freundlich isotherm (R2 = 0.979) and the pseudo-second-order kinetics model (R2 = 0.998). Also, the thermodynamic parameters of the absorption system, such as changes in enthalpy (ΔH = + 9.36 kJ/mol), entropy (ΔS = + 40.72 J/mol k) and Gibbs free energy (ΔG = − 3.5 kJ/mol), were measured and showed that adsorption process of ZnFe2O4@SiO2@Tragacanth gum magnetic nanocomposite was endothermic and spontaneous process. As a result, due to high adsorption capacity, environment friendly, low cost, rapid extraction and non-toxicity, the ZnFe2O4@SiO2@TG magnetic nanocomposite can be used as an effective adsorbent for methylene blue removal from aqueous solutions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, industrial development has led to an ever-increasing rise in industrial wastewater and environmental pollution [1]. Dyes are one of the most important pollutants in various industries such as textiles, paper, plastics, cosmetics and hygiene [2]. Synthetic dyes used in the industry are compounds with complex molecular structure and persist to light, heat and microbial degradation [3]. The discharge of dyed wastewater into the environment, in addition to messing up the beautiful face of nature, disrupts the function of photosynthesis and extirpates the aquatic plants and animals [1]. Also, they may also be carcinogenic or mutagenic [4]. Methylene blue (MB) is a cationic dye that is widely used in the textile industry and is usually toxic and carcinogenic [5, 6]. Burning eyes, nausea, diarrhea and vomiting are also side effects of contact with containing MB sewage [7]. Many techniques, such as coagulation and filtration, ion exchange, filter membranes, adsorption, advanced oxidation and photocatalytic processes, have been widely used for dyes removal. Among these methods, surface adsorption has been widely used due to various advantages such as low cost, easy design and high efficiency [8,9,10,11,12,13,14,15,16]. Many studies have been carried out on the absorption of methylene blue by various adsorbents that can be cited in studies by authors Andreas, Fan, Alehyen Omidi Khaniabadi and Altıntıg such as orange peel [17], tea waste [18], fly ash-based geopolymer [19], aloe vera wastes [5] and magnetic loaded activated carbon adsorbers [6] in 2016–2018.
In recent years, the use of nanoparticles, especially magnetic nanoparticles, as adsorbents for the removal of various pollutants, due to the important properties, including easy separation by an external magnetic field, easy synthesis of nanoparticles, high surface area and ease of enhancement, has been found [12, 20,21,22,23]. Recently, many studies have been conducted on ferrite nanoparticles (NPS) due to important properties such as high surface-to-volume ratio, high stability, recyclability and biocompatibility [24]. Oxidation potential in ferrite NPS is high. For this purpose, it is necessary to modify the surface of ferrite NPS using organic or mineral substances [25]. SiO2 is a non-toxic, water-dispersible and biocompatible substance for coating ferrite NPS [26]. Recently, magnetic NPS have been combined with biopolymers to increase adsorption capacity and have been used for various purposes [27,28,29,30]. Tragacanth gum (TG) as a magnetic biosorbent and porous hydrogel beads is a non-toxic, low-cost, abundant, affordable and biocompatible biopolymer which can be utilized as a thickening agent as well as a binder and even stabilizer [29, 31]. The presence of various functional groups such as primary and secondary hydroxyl groups, carboxylic acid and epoxy group in the catalyst structure provides favorable conditions for reaction with reactants containing functional groups [31]. Iran is the largest producer of quality tragacanth gum [32]. The molecular structure of TG is shown in Fig. 1 [33].
In the present study, the zinc ferrite NPS were synthesized and then a silica layer was fixed on the NPS. Further, the surface of the magnetic NCS was modified with natural TG biocompatible polymer and the application of synthesized magnetic NCS in MB removal was studied. In the present study, the ZnFe2O4@SiO2@Tragacanth gum magnetic nanocomposite as a novel adsorbent was synthesized and was confirmed by FTIR, XRD, TEM, TGA and VSM methods. This novel absorbent has different advantages such as biocompatibility, low toxicity, low cost, high performance in removal, easy availability and easy separation and synthesis.
New nanocomposite due to high absorption capacity compared to some of the above sorbents (Table 1), biocompatibility, easy separation with an external magnetic field, low toxicity, low cost, easy availability and environment friendly of tragacanth (Iran’s largest producer of high-quality tragacanth) is a good absorbent for methylene blue removal.
Parameters such as pH, contact time, initial dye concentration, absorbance dosage, temperature and thermodynamics of the adsorption process were tested. In addition, four isotherm models (Langmuir, Freundlich, Temkin and Dubinin–Radushkevich) and two models of adsorption kinetics (pseudo-first and pseudo-second order) were analyzed. Different techniques such as TEM, XRD, TGA, FTIR and VSM were used to identify NPS.
Materials and methods
Material and Devices
Zinc nitrate (Zn (NO3)2 6H2O), iron nitrate (Fe (NO3)3 9H2O), ethylene diamine (ED), sodium hydroxide (NaOH), ammonia solution 25%, tetraethyl orthosilicate (TEOS), ethanol (EtOH), potassium persulfate (K2S2O8), methyl methacrylate (MMA), ascorbic acid (AA), acetone and methylene blue colored powder were purchased from Merck company and used without purification. Tragacanth gum (TG) was prepared residential natural resources. Stock solution of MB dye was given by dissolving a certain amount of MB in distilled water. ZnFe2O4@SiO2@TG magnetic NCS was characterized by FTIR, XRD, TEM, TGA and VSM patterns. Transmission electron microscopy (TEM) model Zeiss-EM10C-100 kV Germany, Fourier transform infrared spectroscopy (FTIR) model AVATAR370 made in USA, the X-ray diffraction device (XRD) model X’ Pert Pro PANalytical company, UV–Vis spectrophotometer model (UV–Vis spectrophotometer T80 + , PG Instrument Ltd), vibration test sample magnetometer (VSM) model 7400, thermogravimetric analysis (TGA) model STA1500 from Rheumatic scientific company were used as technical devices.
Preparation of the Adsorbent
Synthesis of ZnFe2O4 NPS
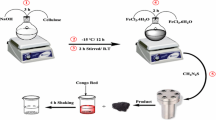
4.9 g of zinc nitrate Zn (NO3)2 and 13.4 g of iron (III) nitrate Fe (NO3)3 were dissolved in 50 mL deionized water (first solution). In the other balloon, 4.2 g of NaOH was dissolved in 70 mL distilled water and 3 mL ethylene diamine (ED) (second solution). Afterward, the first solution was added into the second solution. The solution was heated about 60 min at 90 °C. Then, the solution was washed several times by distilled water and ethanol. Synthesized ZnFe2O4 NPS were put in the vacuum oven, for 7 h and in 80 °C. The powder was calcined for 1 h at 600 °C with a heating rate of 10 °C/min [34].
Synthesis of ZnFe2O4@SiO2 NPS
0.5 g of ZnFe2O4 NPS was added into 20 mL distilled water, 60 mL ethanol and 1 mL ammonia 25%. The mixture obtained was dispersed for 30 min in an ultrasonic bath. 0.5 mL of tetraethyl orthosilicate (TEOS) was mixed with 10 mL ethanol and was added dropwise to the solution of containing NPS. The resulted solution was mixed up for 24 h at room temperature. At last, the solution was washed several times by distilled water and ethanol and NPS were separated by magnet and they were dried in a vacuum oven at 80 °C and for 7 h [25].
Synthesis of ZnFe2O4@SiO2@TG magnetic NCS
Firstly, 1 g of TG was dissolved in 250 mL distilled water at 70 °C in a glass beaker. Then, 2 g of ZnFe2O4@SiO2 NPS was added to the solution under stirring at 1200 rpm. Afterward, 6 mL of methyl methacrylate (MMA) and 1.35 g of ascorbic acid (AA) were added, and the solution was mixed up for 30 min. Then, 1.35 g of K2S2O8 was added to the solution and the solution was mixed up for another 1 h at room temperature. Finally, the sample was washed by acetone for several times and modified NPS were separated by magnet. The sample was dried under vacuum at 60 °C for 6 h [29] (Fig. 2).
Adsorption experiments
All adsorption experiments were performed in laboratory scale and batch system in 250-mL Erlenmeyer flasks containing adsorbents and 50 ml of MB dye with different initial concentrations on a shaker with a stirring speed of 180 rpm. The effect of different parameters such as initial pH of the solution (2–12), initial concentration of dye (10–60 mg/L), contact time (5–120 min), adsorbent dose (0.2–2 g/L) and temperature (15–45 °C) on the removal efficiency and adsorption capacity by ZnFe2O4@SiO2@TG magnetic NCS was studied. At the end of each experiment, the magnetic NCS was separated by an external magnetic field and the remaining dye concentration was measured using UV/Vis spectrophotometer at a wavelength of 665 nm.
Efficiency and capacity of adsorption were calculated by Eqs. (1) and (2):
where C0 and Ce are, respectively, the initial concentration and equilibrium in mg/L, qe is the capacity of dye adsorption in mg/g, m is the mass of adsorbent in g and V is the volume of solution in L [35].
Determination of pHZPC of ZnFe2O4@SiO2@TG magnetic NCS
Fifty milliliters of deionized water was added to Erlenmeyer flasks, and then pH of deionized water in the range of 2 to 12 was set by HCL and NaOH 0.1 N. Afterward, 0.025 g of ZnFe2O4@SiO2@TG magnetic NCS was added to each one of Erlenmeyer flasks and was put on the shaker with 200 rpm for 24 h. After passing 24 h, the final pH of the solution was measured by pH meter. A pH at a point where the initial pH of the solution crossovers the final pH equilibrated by an amount of an adsorbent is related to as the pHZPC.
Results and Discussion
Characteristics of adsorbent
FTIR analysis
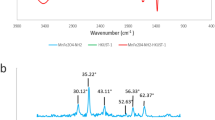
Figure 3 shows the FTIR spectra of ZnFe2O4, ZnFe2O4@SiO2 NPS and ZnFe2O4@SiO2@TG magnetic NCS. In the FTIR spectrum of ZnFe2O4 NPS (Fig. 3a), peaks appeared at wavelengths 453 and 562 cm−1 are, respectively, related to tensile vibrations of Zn–O and Fe–O. As well as, created vibrations in 3405 cm−1 are related to the tensile vibrations of O–H [36, 37]. Furthermore, vibrational peaks in 1087 and 959, respectively, are related to Si–O-Si and Si–OH [38, 39], showing the silica coating on ZnFe2O4 NPS (Fig. 3b). In the FTIR spectra related to ZnFe2O4@SiO2@TG magnetic NCS (Fig. 3c), in addition to listed vibrational peaks, other main vibrational peaks in 2945, 1746 and 1638 cm−1 are, respectively, related to tensile vibrations C–H, C=O and C=C. The adsorption peaks in wavelengths of 1228 and 1138 cm−1 are related to tensile vibrations of C–O [40]. Vibrational bands in (2945, 1746, 1638, 1228 and 1138 cm−1) are reason to fixation of TG cover on ZnFe2O4@SiO2 magnetic NCS.
XRD analysis
Figure 4 shows the XRD patterns of ZnFe2O4, ZnFe2O4@SiO2 NPS and ZnFe2O4@SiO2@TG magnetic NCS. Figure 4a shows the cubic spinel structure of ZnFe2O4 with Fd-3m space group. The medium ZnFe2O4 NPS size was calculated to be about 12 nm according to characteristics of the main peak (311) and using Scherer equation. The XRD pattern of ZnFe2O4@SiO2 NPS (Fig. 4b) showed a broad peak in 2θ = 20–27 which is related to the amorphous of SiO2 shells [26]. Also, the medium ZnFe2O4@SiO2 NPS size was calculated to be about 15 nm. In the XRD pattern of ZnFe2O4@SiO2@TG magnetic NCS (Fig. 4c) presence 2θ in angle of 30.88 relate to amorphous of TG [41]. The medium ZnFe2O4@SiO2@TG magnetic NCS size was calculated to be about 24 nm.
TEM analysis
Figure 5 shows the TEM image of ZnFe2O4, ZnFe2O4@SiO2 NPS and ZnFe2O4@SiO2@TG magnetic NCS with the average size of 13, 17 and 25 nm, respectively, which is similar to the XRD results. Also, the successful coatings of SiO2 and TG on the surface of NPS and cube structure of ZnFe2O4 NPS and branched structure of TG are shown.
VSM analysis
Figure 6 shows the VSM pattern of ZnFe2O4, ZnFe2O4@SiO2 NPS and ZnFe2O4@SiO2@TG magnetic NCS. The VSM patterns of ZnFe2O4 nanoparticle (Fig. 6a) show that these NPS have paramagnetic behavior at room temperature and can be separated with a magnet. Amount of saturated magnetism for ZnFe2O4 NPS equals 19.325 emu/g. With surface modification of ZnFe2O4 NPS by silica (Fig. 6b), yet these NPS at the room temperature show paramagnetic behavior and can be separated with an external magnet. The amount of saturated magnetism for ZnFe2O4@SiO2 NPS equals 15.363 emu/g. Also, the amount of saturated magnetism of ZnFe2O4@SiO2@TG magnetic NCS equals 6.1657 emu/g (Fig. 6c). Although the amount of saturated magnetism has dropped in this magnetic NCS but, this magnetic NCS shows paramagnetic behavior at room temperature and can easily be separated by an external magnet.
TGA analysis
The TGA thermograms of ZnFe2O4, ZnFe2O4@SiO2 NPS and ZnFe2O4@SiO2@TG magnetic NCS are shown in Fig. 7. The TGA of ZnFe2O4 NPS (Fig. 7a) shows a weight loss at 25 to 434 °C of about 1.97% due to evaporation of ethanol, water and crystal structure of ZnFe2O4 NPS [26, 42]. Significant weight loss was observed in the temperature range of 434 to 800 °C. The TGA thermogram of the ZnFe2O4@SiO2 NPS (Fig. 7b) shows the weight loss in two stages. The first weight loss stage in the temperature ranges from 25 to 155 of about (1.45%) due to loss of water present on the surface of the NPS [43]. The two weight loss step ranges between 155 and 450 °C of about 1.25% due to the evaporation water molecules and additional compounds [44]. Significant weight loss was observed in the temperature range of 450 to 800 °C. The thermogram of ZnFe2O4@SiO2@TG magnetic NCS (Fig. 7c) shows the weight loss in four steps. The first weight loss is between 25 and 193 of about 3.2%, due to loss of water, methyl methacrylate and the remaining solvent [28]. Most weight loss in the second stage is between 193 and 390 °C of about 22.37%, due to TG link and methyl methacrylate decomposition [29, 31]. The third step of weight loss is due to decomposition of TG branched structure about 4.55% between 390 and 606 °C [31]. The fourth stage of the weight loss is about 10.43 in the range of 606–800 °C that probably is due to the oxidation of metal oxides [43].
Effect of pH on adsorption
pH is one of the basic parameters that should be considered in analyzing sorbate–sorbent systems and determining the mechanisms of adsorption process, because it affects both the water chemistry and the adsorbent binding sites [45]. In the present study, the effect of pH on the removal of MB dye was investigated in the range of pH = 2–12 with an initial dye concentration of 10 mg/L and adsorbent mass of 0.02 g in 50 mL of coloring solution for 60 min (Fig. 8). With increasing pH from 2 to 8, the efficiency and adsorption capacity increase, so that at pH = 8, the highest removal efficiency and adsorption capacity are observed and after pH = 8, the efficiency and adsorption capacity remain almost constant. The reason for this is related to the adsorbent pHzpc, pka and the MB dye cationic structure. pHzpc is one of the parameters that can be used in the adsorption process. In the present study pHzpc of ZnFe2O4@SiO2@TG magnetic NCS was determined to be 6.86 (Fig. 9). MB is a cationic dye with pka equal to 3.8 [46]. The increase in efficiency in alkaline pH is due to increased hydroxyl (OH−) ions and increasing electrostatic gravity between the positive and negative charges of adsorption sites [47]. On the other hand, considering that pH > pk, MB and pH > pHzpc, consequently, the dominant surface charge on the MB dye surface is positive and the surface load of ZnFe2O4@SiO2@TG magnetic NCS is negative at pH values higher than pHzpc. Therefore, due to the cationic nature of MB dye, the electrostatic gravity of the adsorbent and pollutant increases and the removal efficiency and adsorption capacity increase. In contrast, at pH less than pHzpc adsorbent surface charge and MB dye is positive and by increasing electrostatic repulsion force between the adsorbent and the contaminants removed and adsorption capacity decreases [46, 48]. The results of this study are consistent with the results of studies conducted by Mulugeta and Coskun in 2013 and 2017 [49, 50].
Effects of contact time and concentration on the removal of dye
The effect of contact time on the MB dye adsorption in the range of 5 to 120 min at pH = 8 and 50 mL of MB colored solution with an adsorbent mass of 0.02 g at 10, 20, 30, 40, 50 and 60 mg/L concentrations was tested (Fig. 10). According to the results, with increasing initial dye concentration, the dye adsorption decreases, but the adsorption capacity increases in such a way that the adsorption of MB by ZnFe2O4@SiO2@TG magnetic NCS after 60 min reaches at all concentrations equilibrium. This is due to an increase in the number of dye molecules to bind to existing positions on the surface of the adsorbent and to reduce the inter-particle penetration and to increase the effective collision of MB molecules and adsorbent particles [51]. On the other hand, the adsorption of MB in the early stages of the reaction is rapid, and then it is slowed down near equilibrium, which is due to the abundance of empty surface sites in the early stages of adsorption. With the passage of time, the occupancy of the remaining vacant sites increases due to the repulsive forces between the MB and the adsorbent molecules and, as a result, adsorption decreases [52]. Similar results were observed in 2015 by Seidmohammadi and et al. [47].
Effect of Adsorbent dosage on adsorption
Figure 11 shows the effect of adsorbent dosage on the adsorption capacity and efficiency of MB dye indicated by ZnFe2O4@SiO2@TG magnetic NCS. Increasing the adsorbent dosage from 0.2 to 2 g/L increased the removal of MB because increasing the adsorbent dosage increases the available and active adsorption sites for the interaction between sorbate and sorbent. Also, increasing the amount of adsorbents from 0.2 to 2 g/L led to a decrease in absorbed capacity, because with increasing adsorbent dose, unsaturated residual adsorption sites reduce adsorption capacity during adsorption [53]. The results of studies conducted by Seidmohammadi et al. in 2015 and Gupta et al in 2014 are consistent with the result of study [47, 54].
Adsorption Isotherms
The adsorption isotherms of ZnFe2O4@SiO2@TG magnetic NCS MB dye were investigated, and the data were analyzed by Langmuir and Freundlich, Temkin and Dubinin–Radushkevich equations.
Theoretical Langmuir isotherm describes monolayer adsorption of adsorbate onto a homogeneous adsorbent surface [1]. The linear equation of Langmuir is defined in Eq. (3):
where Ce is the equilibrium concentration of adsorbate (mg/L), qe is the amount of absorbed matter in equilibrium time (mg/g), Kl is the Langmuir constant (L/mg) and qm is the maximum amount of adsorption of amount of adsorbed dye in mg/g monolayer (mg/L).
The main feature of Langmuir equation is separation factor (RL) that is defined in Eq. (4)
where b is Langmuir constant (L/mg), RL is the indicates type of isotherm—favorable adsorption 0 < RL < 1, unfavorable adsorption RL > 1, linear adsorption RL = 1 and irreversible adsorption RL = 0 [3].
The Freundlich isotherm model is based on heterogeneous surface that has unequal energies [55]. Its empirical equation is defined in Eq. (5):
where n is the indicator of the desirability extent of adsorption process and Kf is the amount of capacity of adsorbent adsorption in (mg/g (L/mg)1/n) [35].
In order to estimation of porosity and free energy used to of adsorption, Dubinin Radushkovich model was investigated [56]. The linear equation of this model is defined in Eq. (6):
where qm is the maximum amount of adsorption (mg/g), β (constant of the equation) porosity factor and ε Polanyi potential that is defined by Eq. (7):
where R is the global constant of gas (R = 8.3144 J mol−1 K−1) and T is the temperature (in Kelvin).
The Temkin isotherm model shows between adsorbate/adsorbate interactions, and linear form of Temkin isotherm is given in Eq. (8):
where B is the constant of Temkin (KJ/mol) and AT is the connection constant of Temkin equation (L/g) [56, 57].
According to the isothermal charts (Fig. 12) and the results obtained from Table 1, Freundlich isotherm shows the best interpretation of the MB adsorption using ZnFe2O4@SiO2@TG magnetic NCS. Therefore, the results indicate that the adsorbent level is non-homogeneous or heterogeneous and has unequal and non-uniform energies. The parameter 1/n and n in the Freundlich equation can determine the desirability of the adsorption process. If 1/n is less than 1 and n is between 1 and 10, it indicates that the adsorption process is desirable by Freundlich isotherm model [58]. According to Table 2, the value of n is between 1 and 10; also, the value of 1/n is less than 1, which indicates the desired adsorption of MB using ZnFe2O4@SiO2@TG magnetic NCS The results of studies conducted by some researchers in 2017 are consistent with the present study [59, 60].
Adsorption kinetics models
Kinetic models are used to define the speed of the adsorption process and how this speed controls the equilibrium time. Two kinetic models were studied: pseudo-first order and pseudo-second order. These models are the most used to describe dye (Fig. 13). The pseudo-first-order kinetic model is used more for lower concentrations of solute. The linear forms of pseudo-first-order and pseudo-second-order kinetics are defined in Eqs. (9) and (10):
where qe is the amount of adsorbed MB at equilibrium time (mg/g), qt is the amount of adsorbed MB at time (mg/g), K1 (1/min) is the constant of pseudo-first-order kinetic and K2 (g/mg min) is the constant of pseudo-second-order kinetics [2, 61].
In this study, two kinetic adsorption pseudo-first-order and pseudo-second-order kinetic models have been investigated by kinetics study in different initial concentrations of adsorbent material MB dye using ZnFe2O4@SiO2@TG magnetic NCS. The comparison of the R2 coefficients in the two kinetic models studied shows that the MB dye adsorption process is matched using ZnFe2O4@SiO2@TG magnetic NCS from the pseudo-second-order equation. Also, according to the results obtained (Table 2), the adsorption capacity, which is calculated by considering the kinetic equations (qe, cal), is closer to the adsorption capacity obtained from the adsorption experiment (qe, exp) in the pseudo-second-order kinetic model. This also confirms the adherence of the adsorption process to the pseudo-second-order kinetic model. Therefore, chemical adsorption is a process-limiting step which is suggested to evaluate the reaction speed of this model. In studies conducted in 2017, various researchers reported similar results [62, 63].
The effect of temperature and thermodynamics on adsorption
The experiment with keeping constant of optimal parameters (pH, contact time, adsorbent dosage and dye concentration) was performed at temperatures of 15, 25, 35 and 45 °C. Thermodynamic parameters related to the adsorption process, Gibb’s free energy change (∆G), enthalpy change (∆H) and entropy change (∆S) are determined by Eqs. (11) to (13):
where K indicates the equilibrium adsorption constants of the isotherm fits, R is the universal gas constant (R = 8.3144 J mol−1 K−1) and T is the absolute temperature in Kelvin [53].
Figure 14a shows the effect of temperature on the MB dye adsorption capacity using ZnFe2O4@SiO2@TG magnetic NCS. Based on the results, when the temperature rises from 14 to 45, the adsorption capacity increases, which may be due to increased solubility of the dye with increasing temperature; in addition, it increases the collision between adsorbents and agglomerate and increases the size. The pores are absorbed by the surface and thus increase the adsorption capacity [47].
Thermodynamic parameters including Gibbs free energy (ΔG, kJ/mol), enthalpy (ΔH, KJ/mol) and entropy (ΔS, J.mol−1 K−1) were investigated to describe and confirm the mechanism of absorbing the MB dye using ZnFe2O4@SiO2@TG magnetic NCS (Fig. 14b and Table 3). According to Table 3, ∆G is negative at all temperatures, which indicates self-sustaining adsorption processes. In addition, increasing free energy with increasing temperature indicates that the process is more favorable at higher temperatures. The amount of free energy can also determine the type of physical or chemical adsorption process. If ΔG rate is between 0 and − 20 kJ/mol, the adsorption process is a physical type, but if ΔG is between 80 and − 400 kJ/mol, the adsorption process is a chemical type. When ΔG is less than − 20 kJ/mol, the adsorption process is a physical type. The positive amount of enthalpy also shows that the MB adsorption reaction on the adsorbent is physical and endothermic. If ΔH < 25 kJ/mol, the adsorption reaction is physical and if ∆H > 40 kJ/mol, the adsorption reaction is chemical. Positive entropy values indicate that the disorder of the dye molecules and the adsorbent in the solution increases during the adsorption process of MB on the adsorbent [64, 65] (Table 4).
Conclusion
In this study, ZnFe2O4@SiO2@TG magnetic NCS was synthesized and identified using various methods such as FTIR, XRD, TEM, VSM and TGA. Then, the efficiency of the synthesized magnetic NCS was investigated in the removal of MB from aqueous solutions. Various variables including pH, initial concentration of dye, adsorption dosage, temperature and contact time were tested, and optimum conditions were determined. Under optimal conditions—the pH = 8, the contact time of 60 min, the initial radiation dose of 60 mg/L and the absorbance dose of 0.2 g/L, the maximum adsorption capacity was 109.37 mg/g. In the following, four isotherm models (Langmuir, Freundlich, Temkin and Dubinin–Radushkevich) were investigated in the adsorption of MB using ZnFe2O4@SiO2@TG magnetic NCS, and the balance data were Langmuir isotherm model. The kinetic adsorption analysis showed that pseudo-second-order reactions can be used to predict adsorption kinetics. Thermodynamic parameters showed that the adsorption of MB is self-sustaining. As a result, due to the high absorption capacity of the nanocomposites compared with some absorbents and environment friendly, low cost, rapid extraction and non-toxicity, ZnFe2O4@SiO2@TG magnetic NCS can be used as an effective adsorbent to remove MB from aqueous solutions.
References
Barka N, Abdennouri M, Makhfouk ME (2011) Removal of methylene blue and eriochrome black T from aqueous solutions by biosorption on Scolymus hispanicus L.: kinetics, equilibrium and thermodynamics. J Taiwan Inst Chem Eng 42:320–326
Gusmão KAG, Gurgel LVA, Melo TMS, Gil LF (2012) Application of succinylated sugarcane bagasse as adsorbent to remove methylene blue and gentian violet from aqueous solutions-kinetic and equilibrium studies. Dyes Pigm 92:967–974
Almeida C, Debacher N, Downs A, Cottet L, Mello C (2009) Removal of methylene blue from colored effluents by adsorption on montmorillonite clay. J Colloid Interface Sci 332:46–53
Ali AF, Kovo AS, Adetunji SA (2017) Methylene blue and brilliant green dyes removal from aqueous solution using agricultural wastes activated carbon. J Encapsul Adsorpt Sci 7:95
Rabbani M, Seghatoleslami ZS, Rahimi R (2017) Selective adsorption of organic dye methylene blue by Cs4H2PMo11FeO40 6H2O in presence of methyl orange and rhodamine-B. J Mol Struct
Altıntıg E, Altundag H, Tuzen M, Sarı A (2017) Effective removal of methylene blue from aqueous solutions using magnetic loaded activated carbon as novel adsorbent. Chem Eng Res Des 122:151–163
Arabi S, Sohrabi MR (2014) Removal of methylene blue, a basic dye, from aqueous solutions using nano-zerovalent iron. Water Sci Technol 70:24–31
Alqadami AA, Naushad M, Alothman Z, Ahamad T (2018) Adsorptive performance of MOF nanocomposite for methylene blue and malachite green dyes: kinetics, isotherm and mechanism. J Environ Manag 223:29–36
Sharma G, Naushad M, Pathania D, Kumar A (2016) A multifunctional nanocomposite pectin thorium (IV) tungstomolybdate for heavy metal separation and photoremediation of malachite green. Desalin Water Treat 57:19443–19455
Albadarin AB, Charara M, Tarboush BJA, Ahmad M, Kurniawan TA, Naushad M et al (2017) Mechanism analysis of tartrazine biosorption onto masau stones; a low cost by-product from semi-arid regions. J Mol Liq 242:478–483
Daneshvar E, Vazirzadeh A, Niazi A, Kousha M, Naushad M, Bhatnagar A (2017) Desorption of Methylene blue dye from brown macroalga: effects of operating parameters, isotherm study and kinetic modeling. J Clean Prod 152:443–453
Alqadami AA, Naushad M, Abdalla MA, Khan MR, Alothman ZA (2016) Adsorptive removal of toxic dye using Fe3O4–TSC nanocomposite: equilibrium, kinetic, and thermodynamic studies. J Chem Eng Data 61:3806–3813
Pathania D, Gupta D, Aláa H, Sharma G, Kumar A, Naushad M et al (2016) Photocatalytic degradation of highly toxic dyes using chitosan-g-poly (acrylamide)/ZnS in presence of solar irradiation. J Photochem Photobiol A Chem 329:61–68
Naushad M, Abdullah AL, Othman Z, Rabiul Awual M, Alfadul SM, Ahamad T (2016) Adsorption of rose Bengal dye from aqueous solution by amberlite Ira-938 resin: kinetics, isotherms, and thermodynamic studies. Desalin Water Treat 57:13527–13533
Javadian H, Angaji MT, Naushad M (2014) Synthesis and characterization of polyaniline/γ-alumina nanocomposite: a comparative study for the adsorption of three different anionic dyes. J Ind Eng Chem 20:3890–3900
Gnanasekaran L, Hemamalini R, Naushad M (2018) Efficient photocatalytic degradation of toxic dyes using nanostructured TiO2/polyaniline nanocomposite. Desalin Water Treat 108:322–328
Andreas A, Reinaldo J, Tertira K (2016) A study on the adsorption equilibrium and kinetics of methylene blue onto orange peel wastes as biosorbents. In: International conference of industrial, mechanical, electrical, and chemical engineering (ICIMECE), IEEE, pp 59–62
Liu L, Fan S, Li Y (2018) Removal behavior of methylene blue from aqueous solution by tea waste: kinetics, isotherms and mechanism. Int J Environ Res Public Health 15:1321
El Alouani M, Alehyen S, El Achouri M, Taibi M (2018) Removal of cationic dye-methylene blue-from aqueous solution by adsorption on fly ash-based geopolymer. J Mater Environ Sci 9:32–46
Alqadami AA, Naushad M, Abdalla MA, Ahamad T, ALOthman ZA, Alshehri SM et al (2017) Efficient removal of toxic metal ions from wastewater using a recyclable nanocomposite: a study of adsorption parameters and interaction mechanism. J Clean Prod 156:426–436
Alqadami AA, Naushad M, Alothman ZA, Ghfar AA (2017) Novel metal-organic framework (MOF) based composite material for the sequestration of U (VI) and Th(IV) metal ions from aqueous environment. ACS Appl Mater Interfaces 9:36026–36037
Naushad M, Ahamad T, Al-Maswari BM, Alqadami AA, Alshehri SM (2017) Nickel ferrite bearing nitrogen-doped mesoporous carbon as efficient adsorbent for the removal of highly toxic metal ion from aqueous medium. Chem Eng J 330:1351–1360
Deepa K, Prasad C, Jyothi N, Naushad M, Rajendran S, Karlapudi S et al (2018) Adsorptive removal of Pb(II) metal from aqueous medium using biogenically synthesized and magnetically recoverable core-shell structured AM@ Cu/Fe3O4 nano composite. Desalin Water Treat 111:278–285
Naseri MG, Saion EB, Ahangar HA, Hashim M, Shaari AH (2011) Synthesis and characterization of manganese ferrite nanoparticles by thermal treatment method. J Magn Magn Mater 323:1745–1749
Singh C, Goyal A, Bansal S, Singhal S (2017) SiO2@MFe2O4 core-shell nanostructures: efficient photocatalysts with excellent dispersion properties. Mater Res Bull 85:109–120
Coşkun M, Korkmaz M (2014) The effect of SiO2 shell thickness on the magnetic properties of ZnFe2O4 nanoparticles. J Nanopart Res 16:2316
Hanif S, Shahzad A (2014) Removal of chromium (VI) and dye Alizarin Red S (ARS) using polymer-coated iron oxide (Fe3O4) magnetic nanoparticles by co-precipitation method. J Nanopart Res 16:2429
Mittal A, Ahmad R, Hasan I (2016) Poly (methyl methacrylate)-grafted alginate/Fe3O4 nanocomposite: synthesis and its application for the removal of heavy metal ions. Desalin Water Treat 57:19820–19833
Sadeghi S, Rad FA, Moghaddam AZ (2014) A highly selective sorbent for removal of Cr(VI) from aqueous solutions based on Fe 3 O 4/poly (methyl methacrylate) grafted Tragacanth gum nanocomposite: optimization by experimental design. Mater Sci Eng C 45:136–145
Nasirimoghaddam S, Zeinali S, Sabbaghi S (2015) Chitosan coated magnetic nanoparticles as nano-adsorbent for efficient removal of mercury contents from industrial aqueous and oily samples. J Ind Eng Chem 27:79–87
Sahraei R, Pour ZS, Ghaemy M (2017) Novel magnetic bio-sorbent hydrogel beads based on modified gum tragacanth/graphene oxide: removal of heavy metals and dyes from water. J Clean Prod 142:2973–2984
Hajmohamadi A, Keramat J, Hojjatoleslamy M, Molavi H (2013) Effect of tragacanth gum on texture and staling of commercial sponge cake. J Herb Drugs (Int J Med Herbs) 4:39–42
Khajavi R, Mossavi Pourgharbi SH, Kiumarsi A, Rashidi A (2007) Gum tragacanth fibers from Astragalus gummifer species: effects of influencing factors on mechanical properties of fibers. J Appl Sci 7:2861–2865
Mahmoodi NM (2013) Zinc ferrite nanoparticle as a magnetic catalyst: synthesis and dye degradation. Mater Res Bull 48:4255–4260
Pathania D, Sharma S, Singh P (2013) Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica Bast. Arab J Chem
Nasseri MA, Allahresani A, Raissi H (2014) Mild oxidation of alkenes catalyzed by Fe3O4/SiO2 nanoparticles. React Kinet Mech Catal 112:397–408
Raeisi Shahraki R, Ebrahimi M (2012) Synthesize of superparamagnetic zinc ferrite nanoparticles at room temperature. J Nanostruct 2:413–416
Daffalla SB, Mukhtar H, Shaharun MS (2010) Characterization of adsorbent developed from rice husk: effect of surface functional group on phenol adsorption
Antsiferova Y, Sotnikova N, Parfenyuk E (2013) Different effects of the immunomodulatory drug GMDP immobilized onto aminopropyl modified and unmodified mesoporous silica nanoparticles upon peritoneal macrophages of women with endometriosis. BioMed Res Int
Derrick MR, Stulik D, Landry JM (2000) Infrared spectroscopy in conservation science. Getty Publications, Los Angeles
Mallakpour S, Abdolmaleki A, Tabesh F (2018) Ultrasonic-assisted manufacturing of new hydrogel nanocomposite biosorbent containing calcium carbonate nanoparticles and tragacanth gum for removal of heavy metal. Ultrason Sonochem 41:572–581
Hou X, Wang X, Yao L, Hu S, Wu Y, Liu X (2015) Facile synthesis of ZnFe2O4 with inflorescence spicate architecture as anode materials for lithium-ion batteries with outstanding performance. New J Chem 39:1943–1952
Rameshbabu R, Ramesh R, Kanagesan S, Karthigeyan A, Ponnusamy S (2013) Synthesis of superparamagnetic ZnFe2O4 nanoparticle by surfactant assisted hydrothermal method. J Mater Sci: Mater Electron 24:4279–4283
Kalimuthu K, Rangasamy SC, Rakkiyasamy M (2014) Characterization of ZnFe2O4 nanoparticles obtained by the thermal decomposition. Acta Chim Slov 60:896–900
Yaneva ZL, Georgieva NV (2012) Insights into Congo Red adsorption on agro-industrial materials- spectral, equilibrium, kinetic, thermodynamic, dynamic and desorption studies. A review. Int Rev Chem Eng 4:127–146
Kim JR, Santiano B, Kim H, Kan E (2013) Heterogeneous oxidation of methylene blue with surface-modified iron-amended activated carbon. Am J Anal Chem 4:115
Seidmohammadi A, Asgari G, Leili M, Dargahi A, Mobarakian A (2015) Effectiveness of Quercus branti activated carbon in removal of methylene blue from aqueous solutions. Arch Hygiene Sci 4:217–225
Lai C, Chen C-Y (2001) Removal of metal ions and humic acid from water by iron-coated filter media. Chemosphere 44:1177–1184
Coşkun R, Yıldız A, Delibaş A (2017) Removal of methylene blue using fast sucking adsorbent
Mulugeta M, Lelisa B (2014) Removal of methylene blue (Mb) dye from aqueous solution by bioadsorption onto untreated Parthenium hystrophorous Weed. Mod Chem Appl
Leili M, Ramavandi B (2014) The efficiency evaluation of activated carbon prepared from date stones for removal of methylene blue dye from aqueous solutions
Lian L, Guo L, Guo C (2009) Adsorption of Congo red from aqueous solutions onto Ca-bentonite. J Hazard Mater 161:126–131
Calvete T, Lima EC, Cardoso NF, Vaghetti JC, Dias SL, Pavan FA (2010) Application of carbon adsorbents prepared from Brazilian-pine fruit shell for the removal of reactive orange 16 from aqueous solution: kinetic, equilibrium, and thermodynamic studies. J Environ Manag 91:1695–1706
Gupta VK, Pathania D, Kothiyal N, Sharma G (2014) Polyaniline zirconium (IV) silicophosphate nanocomposite for remediation of methylene blue dye from waste water. J Mol Liq 190:139–145
Dey A, Singh R, Purkait M (2014) Cobalt ferrite nanoparticles aggregated schwertmannite: a novel adsorbent for the efficient removal of arsenic. J Water Process Eng 3:1–9
Erhayem M, Al-Tohami F, Mohamed R, Ahmida K (2015) Isotherm, kinetic and thermodynamic studies for the sorption of mercury (II) onto activated carbon from Rosmarinus officinalis leaves. Am J Anal Chem 6:1
Dada A, Olalekan A, Olatunya A, Dada O (2012) Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J Appl Chem 3:38–45
Naiya T, Bhattacharya A, Das S (2008) Removal of Cd (II) from aqueous solutions using clarified sludge. J Colloid Interface Sci 325:48–56
Dehghani MH, Dehghan A, Alidadi H, Dolatabadi M, Mehrabpour M, Converti A (2017) Removal of methylene blue dye from aqueous solutions by a new chitosan/zeolite composite from shrimp waste: Kinetic and equilibrium study. Korean J Chem Eng 1–9
Nasr JB, Hamdi N, Elhalouani F (2017) Characterization of activated carbon Prepared from sludge paper for methylene blue adsorption
Mahmoodi NM (2013) Nickel ferrite nanoparticle: synthesis, modification by surfactant and dye removal ability. Water Air Soil Pollut 224:1419
Solisio C, Aliakbarian B (2017) Methylene blue adsorption using chabazite: kinetics and equilibrium modelling. Can J Chem Eng
Tang R, Dai C, Li C, Liu W, Gao S, Wang C (2017) Removal of methylene blue from aqueous solution using agricultural residue walnut shell: equilibrium, kinetic, and thermodynamic studies. J Chem
Shakib F, Koohi AD, Pirzaman AK (2017) Adsorption of methylene blue by using novel chitosan-g-itaconic acid/bentonite nanocomposite-equilibrium and kinetic study. Water Sci Technol 75:1932–1943
Mudyawabikwa B, Mungondori HH, Tichagwa L, Katwire DM (2017) Methylene blue removal using a low-cost activated carbon adsorbent from tobacco stems: kinetic and equilibrium studies. Water Sci Technol 75:2390–2402
Abbad B, Lounis A (2014) Removal of methylene blue from colored effluents by adsorption onto ZnAPSO-34 nanoporous material. Desalin Water Treat 52:7766–7775
Chen D, Zeng Z, Zeng Y, Zhang F, Wang M (2016) Removal of methylene blue and mechanism on magnetic γ-Fe2O3/SiO2 nanocomposite from aqueous solution. Water Resour Ind 15:1–13
Inyang M, Gao B, Zimmerman A, Zhang M, Chen H (2014) Synthesis, characterization, and dye sorption ability of carbon nanotube-biochar nanocomposites. Chem Eng J 236:39–46
Abkenar SD, Khoobi M, Tarasi R, Hosseini M, Shafiee A, Ganjali MR (2014) Fast removal of methylene blue from aqueous solution using magnetic-modified Fe3O4 nanoparticles. J Environ Eng 141:04014049
Su H, Li W, Han Y, Liu N (2018) Magnetic carboxyl functional nanoporous polymer: synthesis, characterization and its application for methylene blue adsorption. Sci Rep 8
Acknowledgements
The authors are grateful to the Birjand University of Medical Sciences for financial and technical supports.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Etemadinia, T., Allahrasani, A. & Barikbin, B. ZnFe2O4@SiO2@Tragacanth gum nanocomposite: synthesis and its application for the removal of methylene blue dye from aqueous solution. Polym. Bull. 76, 6089–6109 (2019). https://doi.org/10.1007/s00289-019-02681-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-02681-7