Abstract
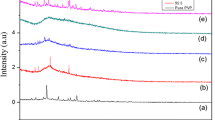
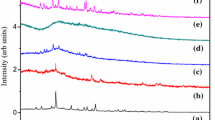
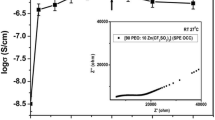
Novel solid polymer electrolyte (SPE) films based on poly(methyl methacrylate) (PMMA) and lithium acetate (CH3COOLi) with different weight ratios of PMMA:CH3COOLi wt% (60:40, 70:30, 80:20 wt%) were prepared by solution casting technique. XRD analysis confirmed the amorphous nature of Li–PMMA SPE films. FTIR analysis revealed the structural changes in polymer by complexation with Li salt. From the optical absorbance studies, the value of lowest energy band gap was found to be 3.06 eV for the composition, PMMA:CH3COOLi (60:40 wt%). From AC impedance studies, the highest value of ionic conductivity 8.21 × 10−5 S/cm at 303 K for the SPE film PMMA:CH3COOLi (60:40 wt%) is observed compared to the reported literature. From the results of Li–PMMA SPE film with high ionic conductivity, it is a promising material for the application of solid-state battery.










Similar content being viewed by others
References
Qingqing Z, Kai L, Fei D, Xingjiang L (2017) Recent advances in solid polymer electrolytes for lithium batteries. Nano Res 10:4139–4174
Scrosati B (2000) Recent advances in lithium ion battery materials. Electro chim Acta 45:2461–2466
Stephan AM (2006) Review on gel polymer electrolytes for lithium batteries. Eur Polym J 42:21–42
Ahmad S (2009) Polymer electrolytes: characteristics and peculiarities. Ionics 15:309–321
Abraham KM, Jiang Z, Carroll B (1997) Highly conductive PEO-like polymer electrolytes. Chem Mater 9:1978–1988
Li Z, Huang J, Liaw BY, Metzler V, Zhang J (2014) A review of lithium deposition in lithium-ion and lithium metal secondary batteries. J Power Sources 254:168–182
Kang X (2004) Non-aqueous liquid electrolytes for lithium-based rechargeable batteries. Chem Rev 104:4303–4418
Armand M (1983) Polymer solid electrolytes–an overview. Solid State Ion 9:745–754
Anil A, Sharma AL (2017) Polymer electrolytes for lithium ion batteries: a critical study. Ionics 23:497–540
Kalhoff J, Eshetu GG, Bresser D, Passerini S (2015) Safer electrolytes for lithium-ion batteries: state of the art and perspectives. Chem Sus Chem 821:54–75
Marcinek M et al (2015) Electrolytes for Li-ion transport–review. Solid State Ion 276:107–126
Baskakova YV, Yarmolenko OV, Efimov ON (2012) Polymer gel electrolytes for lithium batteries. Russ Chem Rev 81:367–380
Berthier C, Gorecki W, Minier M, Armand MB, Chabagno JM, Rigaud P (1983) Microscopic investigation of ionic conductivity in alkali metal salts-poly(ethylene oxide) adducts. Solid State Ion 11:91–95
Cui Y, Xinmiao L, Jingchao C, Zili C, Qinglei W, Weisheng H, Xiaochen L, Zhihong L, Guanglei C, Jiwen F (2017) Facile and reliable in situ polymerization of poly(ethyl cyanoacrylate)-based polymer electrolytes toward flexible lithium batteries. Appl Mater Interfaces 9:8737–8741
Jeddi K, Qazvini NT, Jafari SH, Khonakdar HA (2010) Enhanced ionic conductivity in PEO/PMMA glassy miscible blends: role of nano-confinement of minority component chains. J Polym Sci B Polym Phys 48:2065–2071
Sequeira C, Santos D (2010) Polymer electrolytes fundamentals and applications. Elsevier, Amsterdam
Hellio D, Djabourov MP (2006) Physically and chemically cross linked gelatin gels. Macromol Symp 241:23–27
Armand M, Tarascon JM (2008) Building better batteries. Nature 451:652–657
Song JY, Wang YY, Wan CC (1999) Review of gel-type polymer electrolytes for lithium-ion batteries. J Power Sources 77:183–197
Rao M, Geng X, Liao Y, Hu S, Li W (2012) Preparation and performance of gel polymer electrolyte based on electrospun polymer membrane and ionic liquid for lithium ion battery. J Membr Sci 37:399–400
Kim JK, Shin CR, Ahn JH, Matic A, Jacobsson P (2011) Highly porous LiMnPO4 in combination with an ionic liquid-based polymer gel electrolyte for lithium batteries. Electrochem Commun 13:1105–1108
Stephan AM, Nahm KS, Kulandainathan MA, Ravi G, Wilson J (2006) Poly (vinylidene fluoride-hexafluoropropylene) (PVdF-HFP) based composite electrolytes for lithium batteries. Eur Polym J 42:1728–1734
Alamgir M, Abraham KM (1993) Li ion conductive electrolytes based on poly (vinyl chloride). J Electrochem Soc 140:96
Saito Y, Capigila C, Yamamoto H, Mustarelli P (2000) Ionic conduction mechanisms of polyvinylidene fluoride-hexafluoropropylene type polymer electrolytes with LiN(CF3SO2)2. J Electrochem Soc 147:1645
Panero S, Scrosati B (2000) Gelification of liquid-polymer systems: a valid approach for the development of various types of polymer electrolyte membranes. J Power Sources 90:13–19
Malcom P, Stevens P (2000) Polymer chemistry: an introduction. J Chem Educ 77:167–176
Shukla N, Thakur AK (2009) Role of salt concentration on conductivity optimization and structural phase separation in a solid polymer electrolyte based on PMMA-LiClO4. Ionics 15:357–367
Appetecchi GB, Croce F, Scrosati B (1995) Kinetics and stability of the lithium electrode in poly(methylmethacrylate)-based gel electrolytes. Electrochim Acta 40:991–997
Bohnke O, Frand G, Rezrazi M, Rousselot C, Truche C (1993) Fast ion transport in new lithium electrolytes gelled with PMMA-influence of polymer concentration. Solid State Ion 66:97–104
Wieczorek W, Stevens JR (1997) Impedance spectroscopy and phase structure of polyether–Poly(methyl methacrylate)–LiCF3SO3 blend-based electrolytes. J Phys Chem B 101:1529–1534
Ali AMM, Yahya MZA, Bahron H, Subban RHY, Harun MK, Atan I (2007) Impedance studies on plasticized PMMA-LiX [X: CF3SO3-, N(CF3SO2)2-] polymer electrolytes. Mater Lett 61:2026–2029
Rajendran S, Uma T (2000) Characterization of plasticized PMMA–LiBF4 based solid polymer electrolytes. Bull Mater Sci 2:27–29
Chen HW, Lin TP, Chang FC (2002) Ionic conductivity enhancement of the plasticized PMMA/LiClO4 polymer nano composite electrolyte containing clay. Polymer 43:5281–5288
Senthil J, Nagarajan G, Neyvasagam K (2015) FTIR and ionic conductivity studies on PMMA based gel polymer electrolytes. Int J Enhanc Res Sci Technol Eng 4:76–80
ShahenoorBasha SK, SunitaSundari G, Vijay Kumar K, Rao MC (2017) Optical and dielectric properties of PVP based composite polymer electrolyte films. Polym Sci Ser A 59:554–565
Abdelrazek EM, Hezma AM, El-khodary A, Elzayat AM (2016) Spectroscopic studies and thermal properties of PCL/PMMA biopolymer blend. Egypt J Basic Appl Sci 3:10–15
Aziz Shujahadeen B, Abdulwahid Rebar T, Rsaul Hazhar A, Ahmed Hameed M (2016) In situ synthesis of CuS nanoparticle with a distinguishable SPR peak in NIR region. J Mater Sci Mater Electron 27:4163–4171
Aziz SB, Abdullah OG, Hussein AM, Ahmed HM (2017) From insulating PMMA polymer to conjugated double bond behavior: green chemistry as a novel approach to fabricate small band gap polymers. Polymers 9:626
Ravindra NM, Ganapathy P, Choi J (2007) Energy gap–refractive index relations in semiconductors—an overview. Infrared Phys Technol 50:21–29
Su’ait MS, Ahmada A, Hamzaha H, Rahman MYA (2011) Effect of lithium salt concentrations on blended 49% poly(methyl methacrylate) grafted natural rubber and poly(methyl methacrylate) based solid polymer electrolyte. Electrochim Acta 57:123–131
Uma T, Mahalingam T, Stimming U (2003) Mixed phase solid polymer electrolytes based on poly(methylmethacrylate) systems. Mater Chem Phys 82:478–483
Aziz Shujahadeen B, Rasheed Mariwan A, Hussein Ahang M, Ahmed Hameed M (2017) Fabrication of polymer blend composites based on [PVA-PVP](1 − x):(Ag2S)x(0.01 ≤ x ≤ 0.03) with small optical band gaps: structural and optical Properties. Mater Sci Semicond Process 71:197–203
Krishna Jyothi N, Vijaya Kumar K, Sunita Sundari G, Narayana Murthy P (2016) Ionic conductivity and battery characteristic studies of a new PAN-based Na + ion conducting gel polymer electrolyte system. Indian J Phys 90:289–296
Gurusiddappa J, Madhuri W, Padma Suvarna R, Priya Dasan K (2016) Conductivity and dielectric behavior of polyethylene oxide-lithium perchlorate solid polymer electrolyte films. Indian J Adv Chem Sci 4:4–19
Anil A, Sharma AL, Kumar Dinesh, Sadiq M (2016) Structural and dielectric behaviour of blend polymer electrolyte based on PEO-PAN + LiPF6. Asian J Eng Appl Technol 5:4–7
Acknowledgements
The authors would like to thank the management, Koneru Lakshmaiah Education Foundation (KLEF), for providing kind support for our work. K. Sravanthi is thankful to Dr K. Swapna for UV–visible spectroscopy measurements.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kurapati, S., Gunturi, S.S., Nadella, K.J. et al. Novel solid polymer electrolyte based on PMMA:CH3COOLi effect of salt concentration on optical and conductivity studies. Polym. Bull. 76, 5463–5481 (2019). https://doi.org/10.1007/s00289-018-2659-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2659-5