Abstract
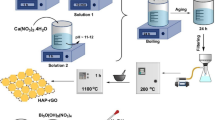
An efficient and novel reduced graphene oxide–polyaniline (rGO–PANI) nanocomposite was used for highly rapid removal of malachite green (MG) from water samples. Graphene oxide was synthesized from graphite by Hummers’ method, and after reduction to reduced graphene oxide by sodium borohydride (NaBH4), its surface was modified by polyaniline to synthesize rGO–PANI nanocomposite. Fourier transform infrared spectrophotometry (FT-IR), electron-dispersive spectroscopy (EDS) and scanning electron microscopy (SEM) were used to characterize the synthesized rGO–PANI nanocomposite. The important parameters affecting the removal efficiency such as pH, contact time and initial MG concentration were studied and optimized. Also, kinetic models including the pseudo-first- and second-order equations were investigated and kinetic parameters of the studied dye were calculated and discussed. It was shown that the adsorption of MG by rGO–PANI nanocomposite could be described by the pseudo-first-order kinetic equation. Also, the experimental isotherms data were analyzed using the Langmuir and Freundlich equations and based on the results, the adsorption of MG by rGO–PANI nanocomposite was followed by both Langmuir and Freundlich equations. The maximum adsorption capacities for MG were calculated and equal to 666.7 mg g−1 which shows that rGO–PANI nanocomposite is a very efficient adsorbent for the removal of MG from water samples.









Similar content being viewed by others
References
Reife A, Reife A, Freeman HS (1996) Environmental chemistry of dyes and pigments. Wiley, New York
Pereira L, Alves M (2012) Dyes—environmental impact and remediation. Springer, New York
Stolz A (2001) Basic and applied aspects in the microbial degradation of azo dyes. Appl Microbiol Biotechnol 56:69–80
Srivastava S, Sinha R, Roy D (2004) Toxicological effects of malachite green. Aquat Toxicol 66(3):319–329
Wang D, Liu L, Jiang X, Yu J, Chen X (2015) Adsorption and removal of malachite green from aqueous solution using magnetic β-cyclodextrin-graphene oxide nanocomposites as adsorbents. Colloids Surf A, Physicochem Engin Aspect 466:166–173
Sun H, Cao L, Lu L (2011) Magnetite/reduced graphene oxide nanocomposites: one step solvothermal synthesis and use as a novel platform for removal of dye pollutants. Nano Res 4(6):550–562
Mall ID, Srivastava VC, Kumar Agarwal N, Mani Mishra I (2005) Adsorptive removal of malachite green dye from aqueous solution by bagasse fly ash and activated carbon-kinetic study and equilibrium isotherm analyses. Colloids Surf A: Physicochem Eng Asp 264:17–28
Ai L, Huang H, Chen Z, Wei X, Jiang J (2010) Activated carbon/CoFe2O4 composites: facile synthesis, magnetic performance and their potential application for the removal of malachite green from water. Chem Engin J 156:243–249
Garg VK, Kumar R, Gupta R (2004) Removal of malachite green dye from aqueous solution by adsorption using agro-industry waste: a case study of Prosopis cineraria. Dyes Pigm 62:1–10
Sartape AS, Mandhare AM, Jadhav VV, Raut PD, Anuse MA, Kolekar SS (2017) Removal of malachite green dye from aqueous solution with adsorption technique using Limonia acidissima (wood apple) shell as low cost adsorbent. Arab J Chem 10:S3229–S3238
Sayğılı H, Güzel F (2015) Performance of new mesoporous carbon sorbent prepared from grape industrial processing wastes for malachite green and congo red removal. Chem Engin Res Design 100:27–38
Sadegh H, Shahryari-ghoshekandi R, Agarwal S, Tyagi I, Asif M, Kumar Gupta V (2015) Microwave-assisted removal of malachite green by carboxylate functionalized multi-walled carbon nanotubes: kinetics and equilibrium study. J Mol Liq 206:151–158
Han R, Wang Y, Sun Q, Wang L, Song J, He X, Dou C (2010) Malachite green adsorption onto natural zeolite and reuse by microwave irradiation. J Hazard Mater 175:1056–1061
Wu X, Wang Y, Liu J, Ma J, Han R (2010) Study of malachite green adsorption onto natural zeolite in a fixed-bed column. Desalin Water Treat 20:228–233
Khairud Dahri M, Rahimi Kooh MR, Kooh LBL (2014) Water remediation using low cost adsorbent walnut shell for removal of malachite green: equilibrium, kinetics, thermodynamic and regeneration studies. J Environ Chem Engin 2:1434–1444
Banerjee S, Sharma GC, Gautam RK, Chattopadhyaya MC, Upadhyay SN, Chandra Sharma Y (2016) Removal of Malachite Green, a hazardous dye from aqueous solutions using Avena sativa (oat) hull as a potential adsorbent. J Mol Liq 213:162–172
Jeyagowri B, Yamuna RT (2016) Potential efficacy of a mesoporous biosorbent Simarouba glauca seed shell powder for the removal of malachite green from aqueous solutions. Desalin Water Treat 57:11326–11336
Chen D, Feng H, Li J (2012) Graphene Oxide: preparation, Functionalization, and Electrochemical Applications. Chem Rev 112(11):6027–6053
Dahaghin Z, Zavvar Mousavi H, Sajjadi SM (2017) Synthesis and application of magnetic graphene oxide modified with 8-Hydroxyquinoline for extraction and preconcentration of trace heavy metal ions. ChemistrySelect 2(3):1282–1289
Eftekhari M, Gheibi M, Akrami M, Iranzad F (2018) Solid-phase extraction of ultra-trace levels of lead using tannic acid-coated graphene oxide as an efficient adsorbent followed by electrothermal atomic absorption spectrometry; response surface methodology-central composite design. New J Chem 42:1159–1168
Sun Z, James DK, Tour JM (2011) Graphene Chemistry: synthesis and Manipulation. J Phys Chem Lett 2:2425–2432
Pei S, Cheng HM (2012) The reduction of graphene oxide. Carbon 50:3210–3228
Yamani K, Berenguer R, Benyoucef A, Morallón E (2018) Preparation of polypyrrole (PPy)-derived polymer/ZrO2 nanocomposites. In press, J Therm Anal Calorim
Iranzad F, Gheibi M, Eftekhari M (2018) Synthesis and application of polythiophene-coated Fe3O4 nanoparticles for preconcentration of ultra-trace levels of cadmium in different real samples followed by electrothermal atomic absorption spectrometry. Inter J Environ Anal Chem 98(1):16–30
Zhan C, Yu G, Lu Y, Wang L, Wujcik E, Wei S (2017) Conductive polymer nanocomposites: a critical review of modern advanced devices. J Mater Chem C 5:1569–1585
Benyakhou S, Belmokhtar A, Zehhaf A, Benyoucef A (2017) Development of novel hybrid materials based on poly(2-aminophenyl disulfide)/silica gel: preparation, characterization and electrochemical studies. J Mol Struct 1150:580–585
Sonkawade RG, Bagal IV, Chodankar NR, Waikar MR, Shinde PS, Shaikh AA (2018) Gamma irradiation: an efficient way to enhance current carrying properties of Ag/Ppy composite. J Mater Sci: Mater Elec 29:11151–11158
Stejskal J, Gilbert RG (2002) Polyaniline. Preparation of a conducting polymer. Pure Appl Chem 74(5): 857-867
Al-Kinani A, Eftekhari M, Gheibi M, Chamsaz M (2018) Polyaniline-coated cerium oxide nanoparticles as an efficient adsorbent for preconcentration of ultra-trace levels of cadmium (II) followed by electrothermal atomic absorption spectrometry. Spectrosc Lett, in press
Chiang JC, MacDiarmid AG (1986) Polyaniline: protonic acid doping of the emeraldine form to the metallic regime. Synth Metals 13(1–3):193–205
Huang WS, Humphrey BD, MacDiarmid AG (1986) Polyaniline, a novel conducting polymer. Morphology and chemistry of its oxidation and reduction in aqueous electrolytes. J Chem Soc, Faraday Trans 82:2385–2400
Daikh S, Zeggai FZ, Bellil A, Benyoucef A (2018) Chemical polymerization, characterization and electrochemical studies of PANI/ZnO doped with hydrochloric acid and/or zinc chloride: differences between the synthesized nanocomposites. J Phys Chem Solids 121:78–84
Pandiselvi K, Thambidurai S (2013) Synthesis of porous chitosan–polyaniline/ZnO hybrid composite and application for removal of reactive orange 16 dye. Colloids Surf A 108:229–238
Eisazadeh A, Eisazadeh H, AnuarKassim K (2013) Removal of Pb(II) using polyaniline composites and iron oxide coated natural sand and clay from aqueous solution. Synth Met 171:56–61
Benykhlef S, Bekhoukh A, Berenguer R, Benyoucef A, Morallon E (2016) PANI-derived polymer/Al2O3 nanocomposites: synthesis, characterization, and electrochemical studies. Colloid Polym Sci 294:1877–1885
Wang Y, Zhang W, Wu X, Luo C, Wang Q, Li J, Hu L (2017) Conducting polymer coated metal-organic framework nanoparticles: facile synthesis and enhanced electromagnetic absorption properties. Synth Met 228:18–24
Shin HJ, Kim KK, Benayad A, Yoon SM, Park HK, Jung IS, Jin MH, Jeong HK, Kim JM, Choi JY, Lee YH (2009) Efficient reduction of graphite oxide by sodium borohydride and its effect on electrical conductance. Adv Funct Mater 19(12):1987–1992
Karandish S, Chamsaz M, Arbab Zavar MH, Gheibi M (2018) Reduced graphene oxide-polyaniline nanocomposite as an efficient adsorbent for solid phase extraction of Co2+ followed by electrothermal atomic absorption spectrometry. Inter J Environ Anal Chem 98(12):1135–1148
Du XS, Zhou CF, Mai YW (2008) Facile synthesis of hierarchical polyaniline nanostructures with dendritic nanofibers as scaffolds. J Phy Chem C 112(50):19836–19840
Ibrahim KA (2017) Synthesis and characterization of polyaniline and poly(aniline-co-o-nitroaniline) using vibrational spectroscopy. Arab J Chem 10:S2668–S2674
Alpat SK, Ozbayrak O, Alpat S, Akcay H (2008) The adsorption kinetics and removal of cationic dye, Toluidine Blue O, from aqueous solution with Turkish zeolite. J Hazard Mater 151:213–220
Santhi T, Manonmani S, Smitha T (2010) Removal of malachite green from aqueous solution by activated carbon prepared from the epicarp of Ricinus communis by adsorption. J Hazard Mater 179:178–186
Onal Y, Akmil-Basar C, Sarici-Ozdemir C (2007) Investigation kinetics mechanisms of adsorption malachite green onto activated carbon. J Hazard Mater 146:194–203
Bulut E, Özacar M, Şengil İA (2008) Adsorption of malachite green onto bentonite: equilibrium and kinetic studies and process design. Microporous Mesoporous Mater 115:234–246
Hammed BH, El-Khaiary MI (2008) Kinetics and equilibrium studies of malachite green adsorption on rice straw-derived char. J Hazard Mater 153:701–708
Acknowledgement
The authors thank the University of Neyshabur for financial support of this project. Also, we appreciated Mrs Melorin Eftekhari and Mahsa Keramati Yazadi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghahramani, A., Gheibi, M. & Eftekhari, M. Polyaniline-coated reduced graphene oxide as an efficient adsorbent for the removal of malachite green from water samples. Polym. Bull. 76, 5269–5283 (2019). https://doi.org/10.1007/s00289-018-2651-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2651-0