Abstract
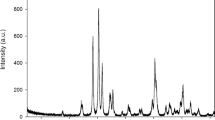
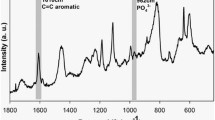
Radiopacity is an important property of dental adhesives, because it allows the adhesive resin to be in contrast with the tooth structure and other restorative materials. This study aimed to develop a radiopaque experimental adhesive resin through the addition of niobium pentoxide (Nb2O5) particles. The effects of adding different concentrations of Nb2O5 nanoparticles synthesized by microwave-assisted hydrothermal synthesis (MHS) were compared to the commercial Nb2O5 microparticles. The experimental adhesive resin was formulated by mixing bisphenol A glycidyl methacrylate (Bis-GMA), 2-hydroxyethyl methacrylate (HEMA), camphorquinone (CQ) and ethyl 4-(dimethylamino)benzoate (EDAB). Experimental adhesive resins were evaluated by the radiopacity, degree of conversion, Knoop microhardness, translucency parameter, depth of cure, viscosity, and sedimentation rate. Synthesized Nb2O5 showed hexagonal structure and nanoneedles aggregates in form of nanoflowers. The incorporation of Nb2O5 nanoparticles into the dental adhesive resin showed high dispersion stability and improved the radiopaque properties.





Similar content being viewed by others
References
Van Landuyt KL, Snauwaert J, De Munck J et al (2007) Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 28:3757–3785. doi:10.1016/j.biomaterials.2007.04.044
Leitune VCB, Collares FM, Trommer RM et al (2013) The addition of nanostructured hydroxyapatite to an experimental adhesive resin. J Dent 41:321–327. doi:10.1016/j.jdent.2013.01.001
Furtos G, Baldea B, Silaghi-Dumitrescu L et al (2013) Influence of inorganic filler content on the radiopacity of dental resin cements. Dent Mater J 31:266–272. doi:10.4012/dmj.2011-225
Schwartz RS, Robbins JW (2004) Post placement and restoration of endodontically treated teeth: a literature review. J Endod 30:289–301. doi:10.1097/00004770-200405000-00001
Schulz H, Schimmoeller B, Pratsinis SE et al (2008) Radiopaque dental adhesives: dispersion of flame-made Ta2O5/SiO2 nanoparticles in methacrylic matrices. J Dent 36:579–587. doi:10.1016/j.jdent.2008.04.010
Moszner N, Salz U (2001) New developments of polymeric dental composites. Prog Polym Sci 26:535–576. doi:10.1016/S0079-6700(01)00005-3
Collares FM, Klein M, Santos PD et al (2013) Influence of radiopaque fillers on physicochemical properties of a model epoxy resin-based root canal sealer. J Appl Oral Sci 21:533–539. doi:10.1590/1679-775720130334
Collares FM, Ogliari FA, Lima GS et al (2010) Ytterbium trifluoride as a radiopaque agent for dental cements. Int Endod J 43:792–797. doi:10.1111/j.1365-2591.2010.01746.x
Collares FM, Leitune VCB, Rostirolla FV et al (2012) Nanostructured hydroxyapatite as filler for methacrylate-based root canal sealers. Int Endod J 45:63–67. doi:10.1111/j.1365-2591.2011.01948.x
Leitune VCB, Takimi A, Collares FM et al (2013) Niobium pentoxide as a new filler for methacrylate-based root canal sealers. Int Endod J 46:205–210. doi:10.1111/j.1365-2591.2012.02107.x
Leitune VCB, Collares FM, Takimi A et al (2013) Niobium pentoxide as a novel filler for dental adhesive resin. J Dent 41:106–113. doi:10.1111/j.1365-2591.2012.02107.x
Ko EI, Weissman JG (1990) Structures of niobium pentoxide and their implications on chemical behavior. Catal Today 8:27–36. doi:10.1016/0920-5861(90)87005-N
Karlinsey RL, Hara AT, Yi K, Duhn CW (2006) Bioactivity of novel self-assembled crystalline Nb2O5 microstructures in simulated and human salivas. Biomed Mater 1:16–23. doi:10.1088/1748-6041/1/1/003
Velten D, Eisenbarth E, Schanne N, Breme J (2004) Biocompatible Nb2O5 thin films prepared by means of the sol-gel process. J Mater Sci Mater Med 15:457–461. doi:10.1023/B:JMSM.0000021120.86985.f7
Bergschmidt P, Bader R, Finze S et al (2011) Comparative study of clinical and radiological outcomes of unconstrained bicondylar total knee endoprostheses with anti-allergic coating. Open Orthop J 5:354–360. doi:10.2174/1874325001105010354
Ding GJ, Zhu YJ, Qi C et al (2015) Porous microspheres of amorphous calcium phosphate: block copolymer templated microwave-assisted hydrothermal synthesis and application in drug delivery. J Colloid Interface Sci 443:72–79. doi:10.1016/j.jcis.2014.12.004
Zhu YJ, Chen F (2014) Microwave-assisted preparation of inorganic nanostructures in liquid phase. Chem Rev 114:6462–6555. doi:10.1021/cr400366s
Leite ER, Vila C, Bettini J, Longo E (2006) Synthesis of niobia nanocrystals with controlled morphology. J Phys Chem B 110:18088–18090. doi:10.1021/jp0642544
Chen M-H (2010) Update on dental nanocomposites. J Dent Res 89:549–560. doi:10.1177/0022034510363765
de Keijser TH, Langford JI, Mittemeijer EJ, Vogels ABP (1982) Use of the Voigt function in a single-line method for the analysis of X-ray diffraction line broadening. J Appl Crystallogr 15:308–314. doi:10.1107/S0021889882012035
Reis LO, Kaizer MR, Ogliari FA et al (2014) Investigation on the use of triphenyl bismuth as radiopacifier for (di)methacrylate dental adhesives. Int J Adhes Adhes 48:80–84. doi:10.1016/j.ijadhadh.2013.09.007
Madruga FC, Ogliari FA, Ramos TS et al (2013) Calcium hydroxide, pH-neutralization and formulation of model self-adhesive resin cements. Dent Mater 29:413–418. doi:10.1016/j.dental.2013.01.004
Melinte V, Buruiana T, Chibac A et al (2016) New acid BisGMA analogs for dental adhesive applications with antimicrobial activity. Dent Mater 32:e314–e326. doi:10.1016/j.dental.2016.09.026
Atai M, Solhi L, Nodehi A et al (2009) PMMA-grafted nanoclay as novel filler for dental adhesives. Dent Mater 25:339–347. doi:10.1016/j.dental.2008.08.005
Le Viet A, Reddy MV, Jose R et al (2011) Electrochemical properties of bare and Ta-substituted Nb2O5 nanostructures. Electrochim Acta 56:1518–1528. doi:10.1016/j.electacta.2010.10.047
Shao R, Cao Z, Xiao Y et al (2014) Enhancing photocatalytic activity by tuning the ratio of hexagonal and orthorhombic phase Nb2O5 hollow fibers. RSC Adv 4:26447–26451. doi:10.1039/c4ra02038c
He J, Hu Y, Wang Z et al (2014) Hydrothermal growth and optical properties of Nb2O5 nanorod arrays. J Mater Chem C 2:8185–8190. doi:10.1039/C4TC01581A
Lopes OF, Paris EC, Ribeiro C (2014) Synthesis of Nb2O5 nanoparticles through the oxidant peroxide method applied to organic pollutant photodegradation: a mechanistic study. Appl Catal B Environ 144:800–808. doi:10.1016/j.apcatb.2013.08.031
Wang X, Chen G, Zhou C et al (2012) N-doped Nb2O5 sensitized by carbon nitride polymer—synthesis and high photocatalytic activity under visible light. Eur J Inorg Chem. doi:10.1002/ejic.201101285
Weissman JG, Ko EI, Wynblatt P, Howe JM (1989) High-resolution electron microscopy and image simulation of TT-, T-, and H-niobia and model silica-supported niobium surface oxides. Chem Mater 1:187–193. doi:10.1021/cm00002a005
Brayner R, Bozon-Verduraz F (2003) Niobium pentoxide prepared by soft chemical routes: morphology, structure, defects and quantum size effect. Phys Chem Chem Phys 5:1457–1466. doi:10.1039/b210055j
Jehng JM, Wachs IE (1991) Structural chemistry and Raman spectra of niobium oxides. Chem Mater 3:100–107. doi:10.1021/cm00013a025
Tiwari JN, Tiwari RN, Kim KS (2012) Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog Mater Sci 57:724–803. doi:10.1016/j.pmatsci.2011.08.003
Cruz AD, Esteves RG, Poiate IAVP et al (2014) Influence of radiopacity of dental composites on the diagnosis of secondary caries: the correlation between objective and subjective analyses. Oper Dent 39:90–97. doi:10.2341/12-377-L
Goshima T, Goshima Y (1990) Radiographic detection of recurrent carious lesions associated with composite restorations. Oral Surg Oral Med Oral Pathol 70:236–239
Aoyagi Y, Takahashi H, Iwasaki N et al (2005) Radiopacity of experimental composite resins containing radiopaque materials. Dent Mater J 24:315–320
Shortall AC, Palin WM, Burtscher P (2008) Refractive index mismatch and monomer reactivity influence composite curing depth. J Dent Res 87:84–88. doi:10.1177/154405910808700115
Tien C-L (2010) Effect of sputtering anisotropic ejection on the optical properties and residual stress of Nb2O5 thin films. Appl Surf Sci 257:481–486
Rodrigues SA, Scherrer SS, Ferracane JL, Della Bona A (2008) Microstructural characterization and fracture behavior of a microhybrid and a nanofill composite. Dent Mater 24:1281–1288. doi:10.1016/j.dental.2008.02.006
Li Y, Swartz ML, Phillips RW et al (1985) Effect of filler content and size on properties of composites. J Dent Res 64:1396–1401. doi:10.1177/00220345850640121501
Krumova M, Klingshirn C, Haupert F, Friedrich K (2001) Microhardness studies on functionally graded polymer composites. Compos Sci Technol 61:557–563. doi:10.1016/S0266-3538(00)00228-1
Say EC, Nakajima M, Senawongse P et al (2006) Microtensile bond strength of a filled vs unfilled adhesive to dentin using self-etch and total-etch technique. J Dent 34:283–291. doi:10.1016/j.jdent.2005.07.003
Sadat-Shojai M, Atai M, Nodehi A, Khanlar LN (2010) Hydroxyapatite nanorods as novel fillers for improving the properties of dental adhesives: synthesis and application. Dent Mater 26:471–482. doi:10.1016/j.dental.2010.01.005
Lee JH, Um CM, Lee IB (2006) Rheological properties of resin composites according to variations in monomer and filler composition. Dent Mater 22:515–526. doi:10.1016/j.dental.2005.05.008
Sadat-Shojai M (2009) Preparation of hydroxyapatite nanoparticles: comparison between hydrothermal and solvo-treatment processes and colloidal stability of produced nanoparticles in a dilute experimental dental adhesive. J Iran Chem Soc 6:386–392. doi:10.1007/BF03245848
Acknowledgements
The authors are grateful to the support of CAPES, CNPq, FAPERGS, CEME-SUL, ClinDoc, and Companhia Brasileira de Metalurgia e Mineração (CBMM) by the ammonium niobium oxalate and Nb2O5 microparticles donations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marins, N.H., Meereis, C.T.W., Silva, R.M. et al. Radiopaque dental adhesive with addition of niobium pentoxide nanoparticles. Polym. Bull. 75, 2301–2314 (2018). https://doi.org/10.1007/s00289-017-2150-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-017-2150-8