Abstract
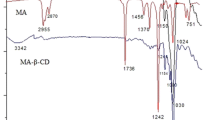
Linalool is a chemical fragrance widely used globally in the cosmetic industry. However, its use has some limitations due to its poor aqueous solubility. Encapsulation of linalool in β-cyclodextrin (β-CD) can improve its solubility. Kinetic and thermodynamic parameters are helpful in understanding the reaction mechanism between a guest molecule and β-CD. In this, we paper evaluated the formation of inclusion complex of linalool in β-CD by electrospray ionization mass spectrometry (ESI–MS) and thermoanalytical methods. We determined the kinetic and thermodynamic parameters, and propose a mechanism of thermal decomposition of the linalool/β-CD inclusion complex. The formation of the inclusion complex was confirmed using ESI–MS, differential scanning calorimetry (DSC) and thermogravimetry (TG). The activation energy of thermal decomposition of the inclusion complex was determined by Flynn–Wall–Ozawa and Starink methods to be 212.16 ± 5.06 and 211.00 ± 4.78 kJ mol−1, respectively, showing there was no strong chemical interaction between linalool and β-CD. The proposed decomposition reaction mechanism was a two-dimensional diffusion model.






Similar content being viewed by others
References
Del Valle EM (2004) Cyclodextrins and their uses: a review. Proc Biochem 39(9):1033–1046
Aprotosoaie AC, Hancianu M, Costache II, Miron A (2014) Linalool: a review on a key odorant molecule with valuable biological properties. Flavour Fragr J 29(4):193–219
Numanoglu U, Sen T, Tarimci N, Kartal M, Koo OMY, Onyuksel H (2007) Use of cyclodextrins as a cosmetic delivery system for fragrance materials: linalool and benzyl acetate. AAPS Pharm Sci Tech 8(4):E1–E9
Menezes PP, Serafini MR, Quintans-Júnior LJ, Silva GF, Oliveira JF, Carvalho FMS, Souza JCC, Matos JR, Alves PB, Matos IL, Hadaruga DI, Araújo AAS (2013) Inclusion complex of (−)linalool and β-cyclodextrin. J Therm Anal Cal 115(3):2429–2437
Song LX, Xu P (2008) A comparative study on the thermal decomposition behaviors between β-cyclodextrin and its inclusion complexes of organic amines. J Phys Chem A 112:11341–11348
Zhu G, Xiao Z, Zhou R, Zhu Y (2014) Study of production and pyrolysis characteristics of sweet orange flavor-β-cyclodextrin inclusion complex. Carbohydr Polm 105:75–80
Higuchi T, Connors KA (1965) Phase-solubility techniques. Adv Anal Chem Instr 4:117–212
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N (2011) ICTAC Kinetics committee recommendations for performing kinetic computations on thermal analysis data. Therm Acta 520:1–19
Ozawa T (1965) A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn 38(11):1881–1886
Starink MJ (1996) A new method for the derivation of activation energies from experiments performed at constant heating rate. Therm Acta 288:97–104
Coats AV, Redfern JP (1964) Kinetic parameters from thermogravimetric data. Nature 201:68–69
Chen Z, Xia Y, Huang Y, Li Y, He Y, Tong Z, Li B (2014) Thermal degradation kinetics study of curcumin with nonlinear methods. Food Chem 155:81–86
Georgieva V, Zvezdova D, Vlaev L (2013) Non-isothermal kinetics of thermal degradation of chitin. J Therm Anal Calorim 111:763–771
Marques HMC (2010) A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Fragr J 25(5):313–326
Meier MM, Luiz MTB, Szpoganicz B, Soldi V (2001) Thermal analysis behavior of beta- and gamma-cyclodextrin inclusion complexes with capric and caprylic acid. Therm Acta 375(1–2):153–160
Xu P, Song LX, Wang HM (2008) Study on thermal decomposition behavior of survived β-cyclodextrin in its inclusion complex of clove oil by nonisothermal thermogravimetry and gas chromatography coupled to time-of-flight mass spectrometry analyses. Therm Acta 469:36–42
Li J-H, Zhang N, Li X-T, Wang J-Y (1997) Thermal stability and decomposition kinetics of the β-CD cinnamic aldehyde inclusion complex. J Incl Phenom Mol Recognit Chem 28:95–103
Li X-T, Li J-H, Zhang G-E, Xi G-X, Lou X-D (1995) Kinetic studies on the thermal dissociation of β-cyclodextrin-anisaldehyde inclusion complex. Therm Acta 262:165–173
Zhang GE, Li X-T, Tian SJ, Li J-H, Lou XD, Cheng QT (1998) Kinetic studies on the thermal dissociation of β-cyclodextrin ethyl benzoate inclusion complexes. J Therm Anal 54:947–956
Cruickshank DL, Rougier NM, Maurel VJ, Rossi RH, Buján EI, Bourne SA, Caira MR (2013) Permethylated β-cyclodextrin/pesticide complexes: X-ray structures and thermogravimetric assessment of kinetic parameters for complex dissociation. J Incl Phenom Mol Recognit Chem 75:47–56
Turmanova SC, Genieva SD, Dimitrova AS, Vlaev LT (2008) Non-isothermal degradation kinetics of filled with rise husk ash polypropene composites. Polm Lett 2(2):133–146
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonetti, P., de Moraes, F.F., Zanin, G.M. et al. Thermal behavior study and decomposition kinetics of linalool/β-cyclodextrin inclusion complex. Polym. Bull. 73, 279–291 (2016). https://doi.org/10.1007/s00289-015-1486-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-015-1486-1