Abstract
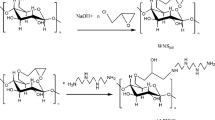
Peanut shell (PNS) was used to prepare a novel adsorbent to achieve resource recovery of the agricultural residue. The hydroxyl groups of peanut shell were turned into –C(CH3)2–Br pendant groups to initiate polymerization of acrylonitrile. Graft copolymer peanut shell/polyacrylonitrile (PNS-g-PAN) was modified by hydroxylamine hydrochloride to transform the cyano groups into amidoxime (AO) groups. The modified copolymer of AO-PNS-g-PAN was used as adsorbent. The maximum adsorption capacity for Hg(II) was 4.45 mmol g−1. The adsorption process fitted well the Freundlich isotherm model and followed pseudo-second-order model. The modified copolymer demonstrated its potential as an efficient adsorbent to solve the problem of Hg(II) contamination.
Graphical abstract
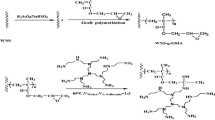
The peanut shell (PNS) macroinitiator was obtained by acylation of hydroxyl groups on the cellulose backbone of the peanut shell and initiated by Cu(0)-mediated RDRP of acrylonitrile. PNS-g-PAN was modified by NH2OH·HCl and used to remove heavy metal ions. The maximum adsorption capacity of Hg(II) was 4.45 mmol g−1.











Similar content being viewed by others
References
Demirbas A (2008) Heavy metal adsorption onto agro-based waste materials: a review. J Hazard Mater 157:220–229
Wan WS, Ngah MA, Hanafiah KM (2008) Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: a review. Bioresour Technol 99:3935–3948
Aydin H, Bulut Y, Yerlikaya CJ (2008) Removal of copper (II) from aqueous solution by adsorption onto low-cost adsorbents. J Environ Manag 87:37–45
Kumar U, Bandyopadhyay M (2006) Sorption of cadmium from aqueous solution using pretreated rice husk. Bioresour Technol 97:104–109
Babarinde NAA, Oyebamiji Babalola J, Adebowale Sanni R (2006) Biosorption of lead ions from aqueous solution by maize leaf. J Phys Sci 1:23–26
Quek SY, Wase DAJ, Forster CF (1998) The use of sago waste for the sorption of lead and copper. Water SA 24:251–256
Zhang BW, Fischer K, Bieniek D, Kettrup A (1994) Synthesis of carboxyl group containing hydrazine-modified polyacrylonitrile fibres and application for the removal of heavy metals. React Polym 24:49–58
Deng SB, Bai RB, Chen JP (2003) Behaviors and mechanisms of copper adsorption on hydrolyzed polyacrylonitrile fibers. J Colloid Interface Sci 260:265–272
Niu YH, Qu RJ, Sun CM, Wang CH, Chen H, Ji CN (2013) Adsorption of Pb(II) from aqueous solution by silica-gel supported hyperbranched polyamidoamine dendrimers. J Hazard Mater 244–245:276–286
Vijayalakshmi A, Arockiasamy DL, Nagendran A, Mohan D (2008) Separation of proteins and toxic heavy metal ions from aqueous solution by CA/PC blend ultrafiltration membranes. Sep Purif Technol 62:32–38
Hawker CJ, Bosman AW, Harth E (2001) New polymer synthesis by nitroxide mediated living radical polymerizations. Chem Rev 101:3661–3688
Moad G, Rizzardo E, Thang SH (2008) Radical addition-fragmentation chemistry in polymer synthesis. Polymer 49:1079–1131
Barner-Kowollik C, Perrier S (2008) The future of reversible addition fragmentation chain transfer polymerization. J Polym Sci Part A: Polym Chem 46:5715–5723
Matyjaszewski K, Jiang X (2001) Atom transfer radical polymerization. Chem Rev 101:2921–2990
Nguyen NH, Percec V (2010) Dramatic acceleration of SET-LRP of methyl acrylate during catalysis with activated Cu(0) wire. J Polym Sci Part A: Polym Chem 48:5109–5119
Percec V, Guliashvili T, Ladislaw JS, Wistrand A, Stjerndahl A, Sienkowska MJ, Monteiro MJ, Sahoo S (2006) Ultrafast synthesis of ultrahigh molar mass polymers by metal-catalyzed living radical polymerization of acrylates, methacrylates, and vinyl chloride mediated by SET at 25 °C. J Am Chem Soc 128:14156–14165
Nguyen NH, Levere ME, Percec V (2012) TREN versus Me6-TREN as ligands in SET-LRP of methyl acrylate. J Polym Sci Part A: Polym Chem 50:35–46
Nguyen NH, Percec V (2011) SET-LRP of methyl acrylate catalyzed with activated Cu(0) wire in methanol in the presence of air. J Polym Sci Part A: Polym Chem 49:4756–4765
Zhai SJ, Wang B, Feng C, Li YJ, Yang D, Hu JH, Lu G, Huang XY (2010) Thermoresponsive PPEGMEA-g-PPEGEEMA well-defined double hydrophilic graft copolymer synthesized by successive SET-LRP and ATRP. J Polym Sci Part A: Polym Chem 48:647–655
Johnson PD, Watson MA, Brown J, Jefcoat IA (2002) Peanut hull pellets as a single use sorbent for the capture of Cu(II) from wastewater. Waste Manag 22:471–480
Edlund U, Albertsson AC (2012) SET-LRP goes “green”: Various hemicellulose initiating systems under non-inert conditions. J Polym Sci Part A: Polym Chem 50:2650–2658
Voepel J, Edlund U, Albertsson AC, Percec V (2011) Hemicellulose-based multifunctional macroinitiator for single-electron-transfer mediated living radical polymerization. Biomacromolecules 12:253–259
de Santa Mariaa LC, Amorimb MCV, Aguiara MRMP, Guimaraesa PIC, Aguiarb MAS, de Costaa AP, Rezende PR, de Carvalho MS, Barbosa FG, Andrade JM, Ribeiro RCC (2001) React. Chemical modification of cross-linked resin based on acrylonitrile for anchoring metal ions. Funct Polym 49:133–143
Zhao T, Zhang LF, Zhang ZB, Zhou NC, Cheng ZP, Zhu XL (2011) A novel approach to modify poly(vinylidene fluoride) via iron-mediated atom transfer radical polymerization using activators generated by electron transfer. J Polym Sci Part A Polym Chem 49:2315–2324
Anirudhan TS, Divya LPS (2009) Kinetic and equilibrium characterization of uranium(VI) adsorption onto carboxylate-functionalized poly(hydroxyethylmethacrylate)-grafted lignocellulosics. J Environ Manag 90:549–560
Liu YH, Zhou WQ, Bai LB, Zhao N, Liu YW (2006) Graft copolymerization of styrene onto casein initiated by potassium diperiodatonickelate (IV) in alkaline medium. J Appl Polym Sci 100:4247–4251
Bai LB, Zhao K, Wu YG, Li WL, Wang SJ, Wang HJ, Ba XW, Zhao HC (2014) A new method for synthesizing hyperbranched polymers with reductive groups using redox/RAFT/SCVP. Chin J Polym Sci 32:385–394
Xiong L, Chen C, Chen Q, Ni JR (2011) Adsorption of Pb(II) and Cd(II) from aqueous solutions using titanate nanotubes prepared via hydrothermal method. J Hazard Mater 189:741–748
Liu CK, Bai RB, Ly QS (2008) Selective removal of copper and lead ions by diethylenetriamine-functionalized adsorbent: behaviors and mechanisms. Water Res 42:1511–1522
Monier M, Abdel-Latif DA (2012) Preparation of cross-linked magnetic chitosan-phenylthiourea resin for adsorption of Hg(II), Cd(II) and Zn(II) ions from aqueous solutions. J Hazard Mater 209–210:240–249
Niu YZ, Qu RJ, Chen H, Mu L, Liu XG, Wang T, Zhang Y, Sun CM (2014) Synthesis of silica gel supported salicylaldehyde modified PAMAM dendrimers for the effective removal of Hg(II) from aqueous solution. J Hazard Mater 278:267–278
Acknowledgments
The finance was supported by the National Natural Science Foundation of China (Nos. 21404051 and 21404052), the Natural Science Foundation of Shandong Province (Nos. ZR2014BQ016 and BS2014CL040), the Talent Introduction Special Funds of Ludong University (Nos. 2014012 and 2014017), the Natural Science Foundation for Distinguished Young Scholars of Shandong province (No. JQ201203).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Bai, L., Wang, D., Chen, H. et al. Synthesis of peanut shell/polyacrylonitrile copolymer via Cu(0)-mediated RDRP and its adsorption behavior after modification. Polym. Bull. 72, 2455–2469 (2015). https://doi.org/10.1007/s00289-015-1423-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-015-1423-3