Abstract
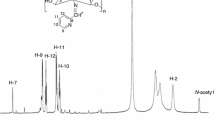
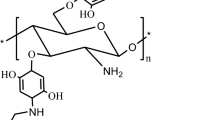
Seven Schiff bases were synthesized from O-carboxymethyl chitosan (CMC) and para-substituted benzaldehydes. The Schiff bases were characterized through Fourier Transform Infrared Spectroscopy, Carbon-13 Nuclear Magnetic Resonance (13C NMR), Distortionless Enhancement of Polarization Transfer (DEPT) 135 NMR, elemental analysis, and acid–base titration. Antibacterial activities of the Schiff bases against Escherichia coli (E. coli, ATCC 35218) and Staphylococcus aureus (S. aureus, ATCC 25923) were measured through the optical density method. Antibacterial activity of the Schiff bases differs from the substituent at the para position of benzaldehyde, and decreases as the sequence OCH3 > CH3 > H > F > Cl > Br > NO2. The IC50 of the Schiff base from 4-methoxylbenzylaldehyde against E. coli and S. aureus is 30 and 34 ppm, respectively, much lower than that of chitosan (53, 48 ppm) and CMC (58, 60 ppm).






Similar content being viewed by others
References
Kang YM, Lee BN, Ko JH, Kim GH, Kang KN, Kim DY, Kim JH, Park YH, Chun HJ, Kim CH, Kim MS (2010) In vivo biocompatibility study of electrospun chitosan microfiber for tissue engineering. Int J Mol Sci 10:4140–4148
VandeVord PJ, Matthew HW, DeSilva SP, Mayton L, Wu B, Wooley PH (2002) Evaluation of the biocompatibility of a chitosan scaffold in mice. J Biomed Mater Res 59:585–590
Ratajska M, Strobin G, Wisniewska-Wrona M, Ciechanska D, Struszczyk H, Boryniec S, Binias D, Binias W (2003) Studies on the biodegradation of chitosan in an aqueous medium. Fibres Text East Eur 11:75–79
Qin CQ, Du YM, Xiao L, Liu Y, Yu HG (2002) Moisture retention and antibacterial activity of modified chitosan by hydrogen peroxide. J Appl Polym Sci 86:1724–1730
Vargas M, González-Martínez C (2002) Recent patents on food applications of chitosan. Recent Pat Food Nutr Agric 2:121–128
Campos M, Cordi LV, Dura N, Mei L (2006) Antibacterial activity of chitosan solutions for wound dressing. Macromol Symp 245–246:515–518
Fabris R, Chow CW, Drikas M (2010) Evaluation of chitosan as a natural coagulant for drinking water treatment. Water Sci Technol 61:2119–2128
Rabea EI, Badawy ME, Stevens CV, Smagghe Guy, Steurbaut W (2003) Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 6:1457–1465
Chung YC, Chen CY (2007) Antibacterial characteristics and activity of acid-soluble chitosan. Biores Technol 99:2806–2814
Chuang YC, Su YP, Chen CC, Jia G, Wang HL, Wu JC, Lin JG (2004) Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacol Sin 25:932–936
Li Zh, Liu XF, Zhuang XP, Guan YL, Yao KD (2002) Manufacture and properties of chitosan/N, O-carboxymethylated chitosan/viscose rayon antibacterial fibers. J Appl Polym Sci 84:2049–2059
Wang JT, Wang HD (2011) Preparation of soluble p-aminobenzoyl chitosan ester by Schiff’s base and Antibacterial activity of the derivatives. Int J Biol Macromol 48:523–529
Liu N, Chen XG, Park HJ, Liu CG, Liu CS, Yu LJ, Meng XH (2006) Effect of MW and concentration of chitosan on antibacterial activity of Escherichia coli. Carbohydr Polym 64:60–66
Kim CH, Jang WC, Heung JC, Kyu SC (1997) Synthesis of chitosan derivatives with quaternary ammonium salt and their antibacterial activity. Polym Bull 38:387–393
Liu XF, Guan YL, Yang DZ, Li Z, Yao KD (2001) Antibacterial action of chitosan and carboxymethylated chitosan. J Appl Polym Sci 79:1324–1335
Xie WM, Xu PX, Wang W, Liu Q (2002) Preparation of water-soluble chitosan derivatives and their antibacterial activity. J Appl Polym Sci 85:1357–1361
Wang XH, Du YM, Fan LH, Liu H, Hu Y (2005) Chitosan–metal complexes as antimicrobial agent: synthesis, characterization and structure-activity study. Polym Bull 55:105–113
Gu CJ, Sun B, Wu WH, Wang FC, Zhu MF (2007) Synthesis, characterization of copper-loaded carboxymethyl-chitosan nanoparticles with effective antibacterial activity. Macromol Symp 254:160–166
Slavica BI, Konstantinovic SS, Savic DS, Veljkovic VB, Gojgic-Cvijov G (2010) The impact of Schiff bases on antibiotic production by Streptomyces hygroscopicus. Med Chem Res 19:690–697
Rehman W, Baloch MK, Muhammad B, Badshah A, Khan KM (2004) Characteristic spectral studies and in vitra anti fungal activity of some Schiff bases and their organotin(IV) complexes. Chin Sci Bull 2:119–122
Varghese S, Muraleedharan Nair MK (2010) Antibacterial and antialgal studies of some lanthanide Schiff base complexes. Int J Appl Bio Pharm Tech 2:608–614
Jin X, Wang JT, Bai J (2009) Synthesis and antibacterial activity of Schiff base from chitosan and citral. Chem Ind Eng Proc 28:2014–2017
Xiao-xia J, Wang JT, Bai J (2010) Synthesis of Schiff base from chitosan and cinnamaldehyde and its antimicrobial activity. J Chem Eng Chinese Univ 24:645–650
Guo ZY, Chen R, Xing R, Liu S, Yu HH, Wang PB, Li CP, Li PC (2006) Novel derivatives of chitosan and their antifungal activities in vitro. Carbohydr Res 341:351–354
Fu XR, Shen Y, Jiang X, Huang D, Yan YQ (2011) Chitosan derivatives with dual-antibacterial functional groups for antimicrobial finishing of cotton fabrics. Carbohydr Polym 85:221–227
Ding CM, Yin PC, Song QP, Li N, Qiao YB (2005) Preparation and characterization of complete deacetylized chitosan. J East China Univ Sci Technol (Nat Sci Ed) 31(3):296–299
Chen XG, Park HJ (2003) Chemical characteristics of O-carboxymethyl chitosans related to the preparation conditions. Carbohydr Polym 53:355–359
Sun T, Xie WM, Xu PX (2004) Superoxide anion scavenging activity of graft chitosan derivatives. Carbohydr Polym 58:379–382
Park JW, Park DM, Park KK (1986) Characterization and metal ion binding properties of carboxymethylchitosan. Polymer (Korea) 10:641–645
Abreu FR, Campana-filho SP (2005) Preparation and characterization of carboxymethylchitosan. Polímeros [online] 2: 79–83
Chen LY, Du YM, Wu HQ, Xiao L (2002) Relationship between molecular structure and moisture-retention ability of carboxymethyl chitin and chitosan. J Appl Polym Sci 83:1233–1241
Liu P, Jia L, Tong QS, Meng XH, Feng YF, Shi JC (2008) Chiral ligands derived from carbohydrates crystal structure of methyl-4, 6-O-benzylidene-3-deoxy-3-(salicylideneamino)-a-d-altropyranoside. Chinese J Struct Chem 9:1119–1122
Young DH, Kauss H (1983) Release of calcium from suspension-cultured Glycine max cells by chitosan, other polycations, and polyamines in relation to effects on membrane permeability. Plant Physiol 73:698–702
Liu XF, Song L, Li L, Li SY, Yao KD (2007) Antibacterial effects of chitosan and its water-soluble derivatives on E. coli, Plasmids DNA, and mRNA. J Appl Polym Sci 103:3521–3528
Qi LF, Xu ZR, Jiang X, Hu CH, Zou XF (2004) Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr Res 339:2693–2700
Hammett LP (1937) The effect of structure upon the reactions of organic compounds benzene derivatives. J Am Chem Soc 59:96
Meneses L, Araya A, Pilaquinga F, Fuentealba P (2008) Relationship between the electrophilicity and σp Hammett constant in Baeyer-Villiger reaction. Chem Phys Lett 460:27–30
Hou HN, Zhu JC, Qi ZD, Zhou B, LiI MY, Liu Y (2010) Antibacterial activity and structure-activity relationships of Schiff bases on Staphylococcus aureus by microcalorimetry. J Wuhan Univ Nat Sci 15:71–77
Acknowledgments
The authors thank the financial support from the National Science Foundation of China (Project No. 50863002), Key Scientific and Technological Project of Haikou (Project No 2010-084), and the “Project 211” of Hainan University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yin, X., Chen, J., Yuan, W. et al. Preparation and antibacterial activity of Schiff bases from O-carboxymethyl chitosan and para-substituted benzaldehydes. Polym. Bull. 68, 1215–1226 (2012). https://doi.org/10.1007/s00289-011-0599-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-011-0599-4