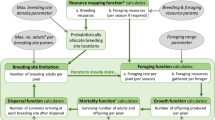
Abstract
The inclusion of cognitive processes, such as perception, learning and memory, are inevitable in mechanistic animal movement modelling. Cognition is the unique feature that distinguishes animal movement from mere particle movement in chemistry or physics. Hence, it is essential to incorporate such knowledge-based processes into animal movement models. Here, we summarize popular deterministic mathematical models derived from first principles that begin to incorporate such influences on movement behaviour mechanisms. Most generally, these models take the form of nonlocal reaction-diffusion-advection equations, where the nonlocality may appear in the spatial domain, the temporal domain, or both. Mathematical rules of thumb are provided to judge the model rationality, to aid in model development or interpretation, and to streamline an understanding of the range of difficulty in possible model conceptions. To emphasize the importance of biological conclusions drawn from these models, we briefly present available mathematical techniques and introduce some existing “measures of success” to compare and contrast the possible predictions and outcomes. Throughout the review, we propose a large number of open problems relevant to this relatively new area, ranging from precise technical mathematical challenges, to more broad conceptual challenges at the cross-section between mathematics and ecology. This review paper is expected to act as a synthesis of existing efforts while also pushing the boundaries of current modelling perspectives to better understand the influence of cognitive movement mechanisms on movement behaviours and space use outcomes.


Similar content being viewed by others
Notes
To save confusion, readers should note that the role of u and v found here are opposite to that found in the original reference to keep the current work as consistent as possible.
References
Amann H (2021) Linear and quasilinear parabolic problems volume I: abstract linear theory. Birkhäuser Basel
An Q, Wang C, Wang H (2020) Analysis of a spatial memory model with nonlocal maturation delay and hostile boundary condition. Discr Contin Dyn Syst-A 40(10):5845–5868
Bharucha-Reid A (1960) Elements of the theory of Markov processes and their applications. McGraw-Hill, New York
Bhattacharyya A (1943) On a measure of divergence between two statistical populations defined by their probability distributions. Bull Calcutta Math Soc 35:99–109
Bracis C, Gurarie E, Bram Van Moorter R (2015) Memory effects on movement behavior in animal foraging. PloS one 10(8):e0136057
Bracis C, Gurarie E, Van Moorter B, Goodwin RA (2015) Memory effects on movement behavior in animal foraging. PLoS One 10:1–21
Britton NF (1990) Spatial structures and periodic traveling waves in an integro-differential reaction diffusion population model. SIAM J Anal Math 50:1663–1688
Buttenschön A, Hillen T (2021) Non-local cell adhesion models: symmetries and bifurcations in 1-D. CMS/CAIMS Books in mathematics
Cantrell R, Cosner C (2004) Spatial ecology via reaction-diffusion equations. Wiley series in mathematical and computational biology. Wiley, New York
Chen S, Yu J (2016) Stability and bifurcations in a nonlocal delayed reaction-diffusion population model. J Differ Equ 260:218–240
Cho E, Kim Y-J (2013) Starvation driven diffusion as a survival strategy of biological organisms. Bull Math Biol 75:845–870
DeAngelis DL, Mooij WM (2005) Individual-based modeling of ecological and evolutionary processes. Annu Rev Ecol Evol Syst 36:147–168
Ellison N, Hatchwell BJ, Biddiscombe SJ, Napper CJ, Potts JR (2020) Mechanistic home range analysis reveals drivers of space use patterns for a non-territorial passerine. J Anim Ecol 89(12):2763–2776
Evans L (1998) Partial differential equations. Graduate studies in mathematics. American Mathematical Society, Washington
Fagan WF, Lewis MA, Auger-Methe M, Avgar T, Benhamou S, Breed G, LaDage L, Schlagel UE, Tang WW, Papastamatiou YP, Forester J, Mueller T (2013) Spatial memory and animal movement. Ecol Lett 16:1316–1329
Fagan WF, Gurarie E, Bewick S, Howard A, Cantrell RS, Cosner C (2017) Perceptual ranges, information gathering, and foraging success in dynamic landscapes. Am Nat 189(5):474–489
Feng C, Lewis MA, Wang C, Wang H (2021) A Fisher-KPP model with a nonlocal weighted free boundary arXiv:2112.00292
Fenton MB (2020) Bats navigate with cognitive maps. Science 369(6500):142
Fieberg J, Signer J, Smith B, Avgar T (2021) ‘How to’ guide for interpreting parameters in habitat-selection analyses. J Anim Ecol 90:1027–1043
Friedman A (1964) Partial differential equations of parabolic type. Prentice-Hall, London
Fronhofer EA, Hovestadt T, Poethke H-J (2013) From random walks to informed movement. Oikos 122(6):857–866
Furter J, Grinfeld M (1989) Local vs. non-local interactions in population dynamics. J Math Biol 27(1):65–80
Giunta V, Hillen T, Lewis M, Potts JR (2022) Local and global existence for nonlocal multispecies advection-diffusion models. SIAM J Appl Dyn Syst 21(3):1686–1708
Giunta V, Hillen T, Lewis MA, Potts J (2022) Detecting minimum energy states and multi-stability in nonlocal advection-diffusion models for interacting species. J Math Biol 56:1–44
Gourley SA, So JW-H (2002) Dynamics of a food-limited population model incorporating nonlocal delays on a finite domain. J Math Biol 44:49–78
Grimm V, Berger U, Bastiansen F, Eliassen S, Ginot V, Giske J, Goss-Custard J, Grand T, Heinz SK, Huse G, Huth A, Jepsen JU, Jorgensen C, Mooij WM, Müller B, Pe’er G, Piou C, Railsback SF, Robbins AM, Robbins MM, Rossmanith E, Rüger N, Strand E, Souissi S, Stillman RA, Vabo R, Visser U, DeAngelis DL (2006) A standard protocol for describing individual-based and agent-based models. Ecol Model 198(1–2):115–126
Gurarie E, Potluri S, Cosner G, Cantrell R, Fagan W (2021) Memories of migrations past: Sociality and cognition in dynamic, seasonal environments. Front Ecol Evol 9:1–15
Hess P (1991) Periodic-parabolic boundary value problems and positivity. John-Wiley and Sons, New York
Hillen T, Painter KJ (2009) A user’s guide to PDE models for chemotaxis. J Math Biol 58(1–2):183–217
Johnston S, Painter K (2019) The impact of short- and long-range perception on population movements. J Theor Biol 460:227–242
Jüngel A, Portisch S, Zurek A (2022) Nonlocal cross-diffusion systems for multi-species populations and networks. Nonlinear Anal 219:112800
Karowe D, Martin M (1989) The effects of quantity and quality of diet nitrogen on the growth, efficiency of food utilization, nitrogen budget, and metabolic rate of fifth-instar spodoptera eridania larvae (lepidoptera: Noctuidae). J Insect Physiol 35:699–708
Keller EF, Segel LA (1970) Initiation of slime mold aggregation viewed as an instability. J Theor Biol 26(3):399–415
Kitchin R, Blades M (2002) The cognition of geographic space, vol 4. I. B Tauris, London
Koy K, Plotnick RE (2007) Theoretical and experimental ichnology of mobile foraging. In: Miller W (ed) Trace fossils. Elsevier, New York, pp 428–441
Lele SR, Keim JL (2006) Weighted distributions and estimation of resource selection probability functions. Ecology 87:3021–3028
Lewis M, Fagan W, Auger-Methe M et al (2021) Learning and animal movement. Front Ecol Evol 9(681704):1–20
Lewis M, Moorcroft PR (2001) ESS analysis of mechanistic models for territoriality: the value of scent marks in spatial resource partitioning. J Theor Biol 210(4):449–461
Lewis MA, Murray JD (1993) Modelling territoriality and wolf-deer interactions. Nature 366:738–740
Lin HY, Fagan WF, Jabin PE (2021) Memory-driven movement model for periodic migrations. J Theor Biol 508:110486
Marley J (2020) Animal selection and movement analysis: Finding missed connections. Master’s thesis, University of Alberta
Moorcroft PR, Lewis MA (2006) Mechanistic home range analysis. Princeton University Press, Princeton
Moorcroft PR, Lewis MA, Crabtree RL (1999) Home range analysis using a mechanistic home range model. Ecology 80:1656–1665
Moser M-B, Rowland DC, Moser EI (2015) Place cells, grid cells, and memory. Cold Spring Harb Perspect Biol 7:a021808
Murray JD (2003) Mathematical biology II: spatial models and biomedical applications, vol 18. Interdisciplinary applied mathematics. Springer, New York
Nathan R, Getz W, Revilla E, Holyoak M, Kadmon R, Saltz D, Smouse PE (2008) A movement ecology paradigm for unifying organismal movement research. PNAS 105(49):19052–19059
Othmer HG, Dunbar SR, Alt W (1988) Models of dispersal in biological systems. J Math Biol 26:263–298
Painter K (2018) Mathematical models for chemotaxis and their applications in self-organisation phenomena. J Theor Biol 481:162–182
Pao CV (1992) FEMS symposium. Springer, New York
Patlak CS (1953) Random walk with persistence and external bias. Bull Math Biophys 15(3):311–338
Pazy A (1983) Semigroups of linear operators and applications to PDEs. Springer-Verlag, Cham
Peacock K (2011) The three faces of ecological fitness. Stud Hist Philos Biol Biomed Sci 42:99–105
Perthame B (2015) Parabolic equations in biology. Lecture notes on mathematical modelling in the life science. Springer, New York
Petrovskii S, Li B (2005) Exactly solvable models of biological invasion. Mathematical and computational biology. Chapman & Hall/CRC. Taylor & Francis, London
Potts J, Giunta V, Lewis MA (2022) Beyond resource selection: emergent spatio-temporal distributions from animal movements and stigmergent interactions. Oikos 6:1–13
Potts JR, Börger L (2023) How to scale up from animal movement decisions to spatiotemporal patterns: an approach via step selection. J Anim Ecol 92(1):16–29
Potts JR, Lewis MA (2016) How memory of direct animal interactions can lead to territorial pattern formation. J R Soc Interface 13:20160059
Potts JR, Lewis MA (2016) Territorial pattern formation in the absence of an attractive potential. J Math Biol 72:25–46
Potts JR, Lewis MA (2019) Spatial memory and taxis-driven pattern formation in model ecosystems. Bull Math Biol 81:2725–2747
Potts JR, Schlägel U (2020) Parametrising diffusion-taxis equations from animal movement trajectories using step selection analysis. Methods Ecol Evol 11:1092–1105
Ranc N, Cagnacci F, Moorcroft PR (2022) Memory drives the formation of animal home ranges: evidence from a reintroduction. Ecol Lett 25(4):716–728
Ranc N, Moorcroft PR, Ossi F, Cagnacci F (2021) Experimental evidence of memory-based foraging decisions in a large wild mammal. PNAS 118:15
Ranta E, Lundberg P, Kaitala V (1999) Resource matching with limited knowledge. Oikos 86:383–385
Renner I, Warton D (2013) Equivalence of MAXENT and Poisson point process models for species distribution modeling in ecology. Biometrics 69:274–281
Riotte-Lambert L, Benhamou S, Chamaillé-Jammes S (2015) How memory-based movement leads to nonterritorial spatial segregation. Am Nat 185(4):E103–E116
Sayama H, Kaufman L, Bar-Yam Y (2006) Spontaneous pattern formation and diversity in spatially structured evolutionary ecology. Focus on biodiversity research. Nova Science Publishers, New York, pp 1–16
Schacter D (1992) Priming and multiple memory systems: perceptual mechanisms of implicit memory. J Cogn Neurosci 4:244–256
Schlägel UE, Lewis MA (2014) Detecting effects of spatial memory and dynamic information on animal movement decisions. Methods Ecol Evol 5(11):1236–1246
Schlagel UE, Merrill EH, Lewis MA (2017) Territory surveillance and prey management: Wolves keep track of space and time. Ecol Evol 7(20):8388–8405
Shi J, Wang C, Wang H (2019) Diffusive spatial movement with memory and maturation delays. Nonlinearity 32:3188–3208
Shi J, Wang C, Wang H (2021) Spatial movement with diffusion and memory-based self-diffusion and cross-diffusion. J Differ Equ 305:242–269
Shi J, Wang C, Wang H, Yan X (2019) Diffusive spatial movement with memory. J Dyn Differ Equ 32:979–1002
Shi Q, Shi J, Wang H (2021) Spatial movement with distributed memory. J Math Biol 82:33
Smith RM, Bain LJ (1975) An exponential power life-testing distribution. Commun Stat 4:469–481
Smouse PE, Focardi S, Moorcroft PR, Kie JG, Forester JD, Morales JM (2010) Stochastic modelling of animal movement. Philos Trans R Soc B: Biol Sci 365(1550):2201–2211
Song Y, Wang H, Wang J (2022) Cognitive consumer-resource dynamics with nonlocal perception. Under Review
Song Y, Wu S, Wang H (2019) Spatiotemporal dynamics in the single population model with memory-based diffusion and nonlocal effect. J Differ Equ 267:6316–6351
Song Y, Wu S, Wang H (2021) Memory-based movement with spatiotemporal distributed delays in diffusion and reaction. Appl Math Comp 404:1–20
Spiegel O, Crofoot MC (2016) The feedback between where we go and what we know—information shapes movement, but movement also impacts information acquisition. Curr Opin Behav Sci 12:90–96
Tang W, Bennet DA (2010) Agent-based modeling of animal movement: a review. Geogr Compass 4(7):682–700
Teitelbaum C, Converse SJ, Fagan W, Böhning-Gaese K, O’Hara R, Lacy A, Mueller T (2016) Experience drives innovation of new migration patterns of whooping cranes in response to global change. Nat Commun 7:12793
Thrun S, Pratt L (1998) Learning to learn. Springer, New York
Toledo S, Shohami D, Schiffner I, Lourie E, Orchan Y, Bartan Y, Nathan R (2020) Cognitive map-based navigation in wild bats revealed by a new high-throughput tracking system. Science 369:188–193
Van Moorter B, Visscher D, Benhamou S, Börger L, Boyce MS, Gaillard J-M (2009) Memory keeps you at home: a mechanistic model for home range emergence. Oikos 118(5):641–652
Volpert V, Petrovskii S (2009) Reaction-diffusion waves in biology. Phys Life Rev 6:267–310
Wang J, Wu S, Shi J (2021) Pattern formation in diffusive predator-prey systems with predator-taxis and prey-taxis. Discr Contin Dyn Syst Series B 3:1273–1289
Wang Z-A (2013) Mathematics of travelling waves in chemotaxis. Discr Contin Dyn Syst Series B 18:601–641
Whitford W, Duval B (2020) Consumers and their effects. In: Whitford WG, Duval BD (eds) Ecology of desert systems, 2nd edn. Academic Press, Cambridge, pp 203–263
Williams HJ, Taylor LA, Benhamou S, Bijleveld AI, Clay TA, de Grissac S, Demšar U, English HM, Franconi N, Gómez-Laich A, Griffiths RC, Kay WP, Morales JM, Potts JR, Rogerson KF, Rutz C, Spelt A, Trevail AM, Wilson RP, Börger L (2020) Optimizing the use of biologgers for movement ecology research. J Anim Ecol 89(1):186–206
Wilmers CC, Nickel B, Bryce CM, Smith JA, Wheat RE, Yovovich V (2015) The golden age of bio-logging: how animal-borne sensors are advancing the frontiers of ecology. Ecology 96(7):1741–1753
Wu J (1996) Theory and applications of partial functional differential equations. Applied Mathematical Sciences. Springer, New York
Wu Z, Yin J, Wang C (2006) Elliptic & parabolic equations. World Scientific, Singapore
Zhao X (2011) Dynamical systems in population biology. CMS books in mathematics. Springer, Cham
Zollner P (2000) Comparing the landscape level perceptual abilities of forest sciurids in fragmented agricultural landscapes. Landscape Ecol 15:523–533
Acknowledgements
We thank our reviewers for their time and effort in providing insightful suggestions and critical feedback, which greatly improved the quality of this manuscript. We also thank our colleagues Peter Thompson and Xiunan Wang for their constructive discussions in creation of Fig. 1 and ultimately catalyzing our motivation to produce an independent document focusing on reaction-advection–diffusion equations. A special thank you to Sophia Salmaniw for painting the graphics used in Fig. 1. Finally, we warmly thank Mark Lewis for his thoughtful feedback and for sharing his wealth of knowledge in the field of mathematical biology. We dedicate this work in honour of his 60th birthday. Hao Wang’s research was partially supported by NSERC Individual Discovery Grant RGPIN-2020-03911 and Discovery Accelerator Supplement Award RGPAS-2020-00090. Yurij Salmaniw was partially supported by NSERC Scholarship PGSD3-535063-2019, President’s Doctoral Prize of Distinction, Josephine Mitchell Research Prize, and multiple Alberta Graduate Excellence Scholarships.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix
A Equivalence of Models
In some cases, the models presented in this review can be reformulated into an equivalent model. We first write the full problem studied in Shi et al. (2019):
in a smooth, bounded domain \(\Omega \), \(\eta (x,t)\) is given initial data, and v(x, t) is defined as
where G is the Green’s function for the heat equation in \(\Omega \) subject to homogeneous Neumann boundary data, \(d_3\) is the diffusion rate for the Green’s function, and \({\mathcal {G}}\) is either the weak or strong kernel defined in (2.27). We first state Lemma 2.1 found in Shi et al. (2021). The lemma is stated as follows.
Lemma A.1
Suppose that kernel \({\mathcal {G}}_w(t)\) is chosen to be the weak kernel defined in (2.27) and define
where G is the Green’s function for the heat equation subject to homogeneous Neumann boundary data. Then,
-
1.
If u(x, t) is the solution of (A.1), then (u(x, t), v(x, t)) is the solution of
$$\begin{aligned} {\left\{ \begin{array}{ll} \frac{\partial u}{\partial t} = d_1 \Delta u + d_2 \nabla \cdot ( u \nabla v) + f(u), \quad \quad x \in \Omega , \ t>0, \\ \frac{\partial v}{\partial t} = d_3 \Delta v + \tau ^{-1} ( u - v), \qquad \qquad \qquad \quad x \in \Omega ,\ t>0, \\ \frac{\partial u}{\partial {\textbf{n}}} = \frac{\partial v}{\partial {\textbf{n}}} = 0 , \qquad \qquad \qquad \qquad \qquad \qquad \quad \,\,\,\, x \in \partial \Omega , \ t>0, \\ u(x,t) = \eta (x,t), \qquad \qquad \qquad \qquad \qquad \quad \,\,\,\, x \in \Omega , \ t \le 0, \\ v(x,0) = \tau ^{-1} \int _{-\infty }^0 \int _\Omega G(x,y,-s) e^{s \tau ^{-1}} \eta (y,s) dy ds. \end{array}\right. } \end{aligned}$$(A.2) -
2.
If (u(x, t), v(x, t)) is a solution of
$$\begin{aligned} {\left\{ \begin{array}{ll} \frac{\partial u}{\partial t} = d_1 \Delta u + d_2 \nabla \cdot ( u \nabla v) + f(u), \quad \quad \qquad x \in \Omega ,\ t\in {\mathbb {R}}, \\ \frac{\partial v}{\partial t} = d_3 \Delta v + \tau ^{-1}(u-v), \qquad \qquad \qquad \qquad \,\,\,\, x \in \Omega , \ t\in {\mathbb {R}}, \\ \frac{\partial u}{\partial {\textbf{n}}} = \frac{\partial v}{\partial {\textbf{n}}} = 0 , \qquad \qquad \qquad \qquad \qquad \qquad \quad \qquad \,\,\,\, x \in \partial \Omega , \ t\in {\mathbb {R}}, \end{array}\right. } \end{aligned}$$(A.3)then u(x, t) satisfies equation (A.1) such that \(\eta (x,s) = u(x,s)\), \(-\infty< s < 0\). In particular, if (u(x), v(x)) is a steady state of (A.3), then u(x) is a steady state of (A.1); if (u(x, t), v(x, t)) is a periodic solution of (A.3), then u(x, t) is a periodic solution of (A.1).
This is an interesting result for two reasons. First, it is interesting to see that model (A.1) is actually equivalent to a Keller-Segel chemotaxis model when the weak kernel is chosen. Second, as a result of this first fact, one can then use the huge body of literature studying chemotaxis models in order to gain insights into this new delay partial differential equation. In the case where one chooses the strong kernel, there is another equivalent system consisting of 3 equations and similar insights can be gathered. This is Lemma 2.2 in Shi et al. (2021), which we do not provide here.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, H., Salmaniw, Y. Open problems in PDE models for knowledge-based animal movement via nonlocal perception and cognitive mapping. J. Math. Biol. 86, 71 (2023). https://doi.org/10.1007/s00285-023-01905-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00285-023-01905-9