Abstract
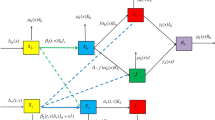
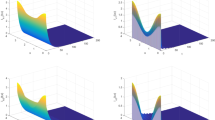
In this paper, we propose a time-periodic reaction–diffusion model which incorporates seasonality, spatial heterogeneity and the extrinsic incubation period (EIP) of the parasite. The basic reproduction number \(\mathcal {R}_0\) is derived, and it is shown that the disease-free periodic solution is globally attractive if \(\mathcal {R}_0<1\), while there is an endemic periodic solution and the disease is uniformly persistent if \(\mathcal {R}_0>1\). Numerical simulations indicate that prolonging the EIP may be helpful in the disease control, while spatial heterogeneity of the disease transmission coefficient may increase the disease burden.



Similar content being viewed by others
References
Abboubakar H, Buonomo B, Chitnis N (2016) Modelling the effects of malaria infection on mosquito biting behaviour and attractiveness of humans. Ricerche Mat 65:329–346
Bacaër N, Guernaoui S (2006) The epidemic threshold of vector-borne diseases with seasonality. J Math Biol 53:421–436
Buonomo B, Vargas-De-León C (2013) Stability and bifurcation analysis of a vector-bias model of malaria transmission. Math Biosci 242:59–67
Chamchod F, Britton NF (2011) Analysis of a vector-bias model on malaria transmission. Bull Math Biol 73:639–657
Cosner C, Beier JC, Cantrell RS, Impoinvil D, Kapitanski L, Potts MD, Troyo A, Ruan S (2009) The effects of human movement on the persistence of vector-borne diseases. J Theor Biol 258:550–560
Daners D, Medina PK (1992) Abstract evolution equations, periodic problems and applications, Pitman research notes in mathematics series, vol 279. Longman, Harlow
Esteva L, Vargas C (1998) Analysis of a dengue disease transmission model. Math Biosci 150:131–151
Ewing DA, Cobbold CA, Purse BV, Nunn MA, White SM (2016) Modelling the effect of temperature on the seasonal population dynamics of temperate mosquitoes. J Theor Biol 400:65–79
Forouzannia F, Gumel AB (2014) Mathematical analysis of an age-structured model for malaria transmission dynamics. Math Biosci 247:80–94
Friedman A (1964) Partial differential equations of parabolic type. Prentice-Hall, Englewood Cliffs
Grassly NC, Fraser C (2006) Seasonal infectious disease epidemiology. Proc R Soc B 273:2541–2550
Gutierrez JB, Galinski MR, Cantrell S, Voit EO (2015) From within host dynamics to the epidemiology of infectious disease scientific overview and challenges. Math Biosci 270:143–155
Hay SI, Were EC, Renshaw M, Noor AM, Ochola SA, Olusanmi I, Alipui N, Snow RW (2003) Forecasting, warning, and detection of malaria epidemics: a case study. Lancet 361:1705–1706
Hosack GR, Rossignol PA, van den Driessche P (2008) The control of vector-borne disease epidemics. J Theor Biol 255:16–25
Kingsolver JG (1987) Mosquito host choice and the epidemiology of malaria. Am Nat 130:811–827
Lacroix R, Mukabana WR, Gouagna LC, Koella JC (2005) Malaria infection increases attractiveness of humans to mosquitoes. PLoS Biol 3:1590–1593
Liang X, Zhao X-Q (2007) Asymptotic speeds of spread and traveling waves formonotone semiflows with applications. Commun Pure Appl Math 60:1–40
Liang X, Zhang L, Zhao X-Q (2017) Basic reproduction ratios for periodic abstract functional differential equations (with application to a spatial model for Lyme disease). J Dyn Differ Equ. https://doi.org/10.1007/s10884-017-9601-7
Lou Y, Zhao X-Q (2010) A climate-based malaria transmission model with structured vector population. SIAM J Appl Math 70:2023–2044
Lou Y, Zhao X-Q (2011) A reaction–diffusion malaria model with incubation period in the vector population. J Math Biol 62:543–568
Macdonald G (1957) The epidemiology and control of malaria. Oxford University Press, London
Magal P, Zhao X-Q (2005) Global attractors and steady states for uniformly persistent dynamical systems. SIAM J Math Anal 37:251–275
Martin RH, Smith HL (1990) Abstract functional differential equations and reaction–diffusion systems. Trans Am Math Soc 321:1–44
Metz JAJ, Diekmann O (1986) The dynamics of physiologically structured populations. Springer, New York
Niger AM, Gumel AB (2008) Mathematical analysis of the role of repeated exposure on malaria transmission dynamics. Differ Equ Dyn Syst 16:251–287
Okuneye K, Gumel AB (2017) Analysis of a temperature- and rainfall-dependent model for malaria transmission dynamics. Math Biosci 287:72–92
Ross R (1911) The prevention of malaria, 2nd edn. Murray, London
Smith HL (1995) Monotone dynamical systems: an introduction to the theory of competitive and cooperative systems, mathematical surveys and monographs, vol 41. American Mathematical Society, Providence
Smith DL, Dushoff J, McKenzie FE (2004) The risk of a mosquito-borne infection in a heterogeneous environment. PLoS Biol 2:1957–1964
Tatem AJ, Hay SI, Rogers DJ (2006) Global traffic and disease vector dispersal. Proc Natl Acad Sci USA 103:6242–6247
Thieme HR (2009) Spectral bound and reproduction number for infinite-dimensional population structure and time heterogeneity. SIAM J Appl Math 70:188–211
Vargas-De-León C (2012) Global analysis of a delayed vector-bias model for malaria transmission with incubation period in mosquitoes. Math Biosci Eng 9:165–174
Wang X, Zhao X-Q (2017) A periodic vector-bias malaria model with incubation period. SIAM J Appl Math 77:181–201
Wu J (1996) Theory and applications of partial functional differential equations. Springer, New York
Xiao Y, Zou X (2014) Transmission dynamics for vector-borne diseases in a patchy environment. J Math Biol 69:113–146
Xu Z, Zhao X-Q (2012) A vector-bias malaria model with incubation period and diffusion. Discrete Contin Dyn Syst Ser B 17:2615–2634
Zhang L, Wang Z, Zhao X-Q (2015) Threshold dynamics of a time periodic reaction–diffusion epidemic model with latent period. J Differ Equ 258:3011–3036
Zhao X-Q (2017a) Basic reproduction ratios for periodic compartmental models with time delay. J Dyn Differ Equ 29:67–82
Zhao X-Q (2017b) Dynamical systems in population biology, 2nd edn. Springer, New York
Acknowledgements
We are grateful to two anonymous referees for careful reading and valuable comments which led to improvements of our original manuscript. We also sincerely thank Lei Zhang for his helpful discussions on the numerical computation of \(\mathcal {R}_0\).
Author information
Authors and Affiliations
Corresponding author
Additional information
Bai’s research was supported by NSF of China (11401453); Peng’s research was supported by NSF of China (Nos. 11671175, 11271167, 11571200), the Priority Academic Program Development of Jiangsu Higher Education Institutions, Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (No. PPZY2015A013) and Qing Lan Project of Jiangsu Province; and Zhao’s research was supported in part by the NSERC of Canada.
Rights and permissions
About this article
Cite this article
Bai, Z., Peng, R. & Zhao, XQ. A reaction–diffusion malaria model with seasonality and incubation period. J. Math. Biol. 77, 201–228 (2018). https://doi.org/10.1007/s00285-017-1193-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-017-1193-7
Keywords
- Vector-bias malaria model
- Seasonality
- Incubation period
- Basic reproduction number
- Threshold dynamics
- Periodic solution