Abstract
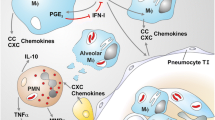
The immune response to Mycobacteriumtuberculosis (Mtb) infection is the formation of multicellular lesions, or granolomas, in the lung of the individual. However, the structure of the granulomas and the spatial distribution of the immune cells within is not well understood. In this paper we develop a mathematical model investigating the early and initial immune response to Mtb. The model consists of coupled reaction-diffusion-advection partial differential equations governing the dynamics of the relevant macrophage and bacteria populations and a bacteria-produced chemokine. Our novel application of mathematical concepts of internal states and internal velocity allows us to begin to study this unique immunological structure. Volume changes resulting from proliferation and death terms generate a velocity field by which all cells are transported within the forming granuloma. We present numerical results for two distinct infection outcomes: controlled and uncontrolled granuloma growth. Using a simplified model we are able to analytically determine conditions under which the bacteria population decreases, representing early clearance of infection, or grows, representing the initial stages of granuloma formation.
Similar content being viewed by others
References
Bloom, B.R.: Tuberculosis: pathogenesis, protection, and control. ASM Press, Washington, DC, 1994
Chiu, C., Hoppensteadt, F.C.: Mathematical models and simulations of bacterial growth and chemotaxis in a diffusion gradient chamber. J. Math. Biol. 42, 120–144 (2001)
Collins, H.L., Kaufmann, S.H.E.: The many faces of host responses to tuberculosis. Immunol. 103, 1–9 (2001)
Comstock, G.W., Livesay, V.T., Woolpert, S.F.: The prognosis of a positive tuberculin reaction in childhood and adolescence. Am. J. Epidemiol. 99, 131–138 (1974)
Dannenberg, A.M., Rook, G.S.W.: Pathogenesis of pulmonary tuberculosis: an interplay of tissue-damaging and macrophage-activating immune responses - dual mechanisms that control bacillary multiplication. In: Bloom,~B.R., (ed) Tuberculosis: pathogenesis, protection, and control. ASM Press, Washington, DC, 1994
DesJardin, L.E., Kaufman, T.M., Potts, B., Kutzbach, B., Yi, H., Schlesinger, L.S.: Mycobacterium tuberculosis-infected human macrophages exhibit enhanced cellular adhesion with increased expression of LFA-1 and ICAM-1 and reduced expression and/or function of complement receptors, FcγRii and the mannose receptor. Microbiol. 148, 3161–3171 (2002)
Emile, J.-.F, Patey, N., Altare, F., Lamhamedi, S., Jouanguy, E., Boman, F., Quillard, J., Lecomte-Houcke, M., Verola, O., Mousnier, J.-F., Dijoud, F., Blanche, S., Fischer, A., Brousse, N., Casanova, J.-L.: Correlation of granuloma structure with clinical outcome defines two types of idiopathic disseminated BCG infection. J. Pathology 181, 25–30 (1997)
Flesch, I.E., Kaufmann, S.H.: Activation of tuberculostatic macrophage functions by gamma interferon, interleukin-4, and tumor necrosis factor. Infect. Immun. 58, 2675–2677 (1990)
Flynn, J.L., Chan, J.: Immunology of tuberculosis. Annu. Rev. Immunol. 19, 93–129 (2001)
Flynn, J.L., Chan, J.: Tuberculosis: latency and reactivation. Infect. Immun. 69, 4195–4201 (2001)
Höfer, T., Sherratt, J.A., Maini, P.K.: Cellular pattern formation during Dictyostelium aggregation. Phy. D 85, 425–444 (1995)
Jackson, T.L., Byrne, H.M.: A mathematical model to study the effects of drug resistance and vasculature on the response of solid tumors to chemotherapy. Math. Biosci. 164, 17–38 (2000)
Janeway, C.A., Travers, P., Walport, M., Shlomchik, M.: Immuno biology. Garland, New York, 1994
Keller, E.F., Segel, L.A.: Model for chemotaxis. J. theor. Biol. 30, 225–234 (1971)
Keller, E.F., Segel, L.A.: Traveling bands of chemotactic bacteria: a theoretical analysis. J. theor. Biol. 30, 235–248 (1971)
Kirschner, D., Perelson, A.: A model for the immune system response to HIV: AZT treatment studies. In: Axelrod, A.O., Kimmel, D., and Langlais, M., (eds) Mathematical Population Dynamics: Analysis of Hetergeneity and Theory of Epidemics, Wuerz Publishing, p. 295–310 (1995)
Lauffenburger, D.A., Linderman, J.J.: Receptors: models for binding, trafficking, and signaling. Oxford University Press, New York, 1993
Lev Bar Or, R.: Feedback mechanisms between T helper cells and macrophages in the determination of the immune response. Math. Biosci. 163, 35–58 (2000)
Lin, Y., Zhang, M., Barnes, P.F.: Chemokine production by a human alveola epithelial cell line in response to mycobacterium tuberculosis. Infect. Immun. 66, 1121–1126 (1998)
Mayanja-Kizza, H., Johnson, J.L., Hirsch, C.S., Peters, P., Surewicz, K., Wu, M., Nalugwa, G., Mubiru, F., Luzze, H., Wajja, A., Aung, H., Ellner, J.J., Whalen, C., Toossi, Z.: Macrophage-activating cytokines in human immunodeficiency virus type 1-infected and -uninfected patients with pulmonary tuberculosis. J. Infect. Dis. 183, 1805–1809 (2001)
Mims, C., Dimmock, N., Nash, A., Stephen, J.: Mims’ Pathogenesis of Infectious Disease. Academic Press, New York, 1995
Myerscough, M.R., Maini, P.K., Painter, K.J.: Pattern formation in a generalized chemotactic model. Bull. Math. Biol. 60, 1–26 (1998)
NAG Ltd.: NAG Fortran Library Manual, Mark 19 edition, 1999
Nathan, C.F., Murray, H.W., Wiebe, M.E., Rubin, B.Y.: Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 158, 670–689 (1983)
Owen, M.R., Sherratt, J.A.: Pattern formation and spatiotemporal irregularity in a model for macrophage-tumour interactions. J. theor. Biol. 189, 63–80 (1997)
Owen, M.R., Sherratt, J.A.: Mathematical modelling of macrophage dynamics in tumours. Math. Models Methods Appl. Sci. 9, 513–539 (1999)
Painter, K.J., Maini, P.K., Othmer, H.G.: Development and applications of a model for cellular respose to multiple chemotactic cues. J. Math. Biol. 41, 285–314 (2000)
Pan, Z.K., Chen, L.-Y., Cochrane, C.G., Zuraw, B.L.: fMet-Leu-Phe stimulates proinflammatory cytokine gene expression in human peripheral blood monocytes: the role of phosphatidylinositol 3-kinase. J. Immunol. 164, 404–411 (2000)
Pilyugin, S.S.: Modeling immune responses with handling time. Bull. Math. Biol. 62, 869–890 (2000)
Sannomiya, P., Craig, R.A., Clewell, D.B., Suzuki, A., Fujino, M., Till, G.O., Marasco, W.A.: Characterization of a class of nonformylated enterococcus faecalis-derived neutrophil chemotactic peptides: the sex pheromones. Proc. Natl. Acad. Sci. USA 87, 66–70 (1990)
Saukkonen, J.J., Bazydlo, B., Thomas, M., Strieter, R.M., Keane, J., Kornfeld, H.: β-chemokines are induced by mycobacterium tuberculosis and inhibit its growth. Infect. Immun. 70, 1684–1693 (2002)
Schluger, N.W., Rom, W.N.: The host immune response to tuberculosis. Am. J. Respir. Crit. Care Med. 157, 679–691 (1998)
Sherratt, J.A.: Chemotaxis and chemokinesis in eukaryotic cells: The Keller-Segel equations as an approxination to a detailed model. Bull. Math. Biol. 56, 129–146 (1994)
Sherratt, J.A., Sage, E.H., Murray, J.D.: Chemical control of eukaryotic cell movement: A new model. J. theor. Biol. 162, 23–40 (1993)
Sozzani, S., Luini, W., Molino, M., Jílek, P., Bottazzi, B., Cerletti, C., Matsushima, K., Mantovani, A.: The signal transduction pathway involved in the migration induced by a monocyte chemotactic cytokine. J. Immunol. 147, 2215–2221 (1991)
Stout, R.D., Bottomly, K.: Antigen-specific activation of effector macrophages by IFN-gamma producing (TH1) t cell clones. failure of IL-4-producing (TH2) t cell clones to activate effector function in macrophages. J. Immunol. 142, 760–765 (1989)
Tran, C.L., Jones,~A.D., Donaldson, K.: Mathematical model of phagocytosis and inflammation after the inhalation of quartz at different concentrations. Scand. J. Work Environ. Health 21, 50–54 (1995)
van Crevel, R., Ottenhoff, T.H.M., van der Meer, J.W.M.: Innate immunity to mycobacterium tuberculosis. Clin. Microbiol. Rev. 15, 294–309 (2002)
Ward, J.P., King, J.R.: Mathematical modelling of avascular-tumour growth. IMA J. Math. Appl. Med. Biol. 14, 39–69 (1997)
Ward, J.P., King, J.R.: Mathematical modelling of avascular-tumour growth ii: Modelling growth saturation. IMA J. Math. Appl. Med. Biol. 16, 171–211 (1999)
WHO.: WHO Report 2001: Global Tuberculosis Control. Technical report, World Health Organization, 2001
Wigginton, J.E., Kirschner, D.: A model to predict cell-mediated immune regulatory mechanisms during human infection with mycobacterium tuberculosis. J. Immunol. 166, 1951–1967 (2001)
Wodarz, D., Lloyd, A.L., Jansen, V.A.A., Nowak, M.A.: Dynamics of macrophage and T cell infection by HIV. J. theor. Biol. 196, 101–113 (1999)
Author information
Authors and Affiliations
Additional information
Send offprint requests to:kirschne@umich.edu
Rights and permissions
About this article
Cite this article
Gammack, D., Doering, C. & Kirschner, D. Macrophage response to Mycobacteriumtuberculosis infection. J. Math. Biol. 48, 218–242 (2004). https://doi.org/10.1007/s00285-003-0232-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-003-0232-8