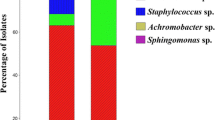
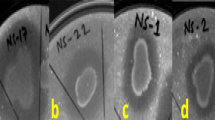
Abstract
Root nodules of legume plants are devoted for hosting endophytic symbiotic bacteria that fix atmospheric nitrogen but recently proved as a niche for various non-rhizobial endophytes (NRE) also. In the present investigation, one rhizobial and two NRE were isolated and characterized as Rhizobium sp. AAU B3, Bacillus sp. AAU B6 and Bacillus sp. AAU B12. These isolates were studied for in vitro biocontrol activity against two pathogenic fungi. NRE isolates exhibited antifungal activity against root rot causing Macrophomina phaseolina (ITCC-6749) isolated from Vigna radiata and wilt causing pathogen Fusarium udum Butler isolated from Cajanus cajan in liquid as well as on solid medium. Furthermore, their antagonism was increased markedly when combined with Rhizobium sp. Moreover, Bacillus sp. AAU B6 showed amplification of the zwittermicin A gene (~ 950 bp) which is evident for the production of antibiotics. All three isolates showed HCN production in vitro also, Bacillus sp. AAU B12 exhibited amplification of its gene hcnC. Pathogenic fungal hyphae became thin, transparent, and bent as well as fungi lost their normal growth and branching patterns when exposed to volatile compounds produced by NRE. All the 3 isolates produced siderophores, however siderophore production was increased considerably when all three strains are mixed together. Furthermore, all the three isolates produced cell wall degrading enzymes (chitinase, protease, and cellulase) but lipolytic activity was exhibited only by Rhizobium sp. AAU B3. When NRE inoculated in combination of Rhizobium; overcomes the disease severity against M. phaseolina under pot study. Thus, from present study it is concluded that co-inoculation of NRE and Rhizobium sp. can be exploited as biocontrol bio-agents against M. phaseolina in green gram at field levels.





Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Dahmani MA, Desrut A, Moumen B, Verdon J, Mermouri L, Kacem M, Coutos-Thévenot P, Kaid-Harche M, Bergès T, Vriet C (2020) Unearthing the plant growth- promoting traits of Bacillus megaterium RmBm31, an endophytic bacterium isolated from root nodules of Retama monosperma. Front Plant Sci 11:124. https://doi.org/10.3389/fpls.2020.00124
Hansen BL, Pessotti RC, Fischer MS, Collins A, El-Hifnawi L, Liu MD, Traxlera MF (2020) Cooperation, competition, and specialized metabolism in a simplified root nodule microbiome. Bio 11:e01917-e1920. https://doi.org/10.1128/mBio.01917-20
Zhao LF, Xua YJ, Lai XH (2018) Antagonistic endophytic bacteria associated with nodules of soybean (Glycine max L.) and plant growth-promoting properties. Braz J Microbiol 49:269–278. https://doi.org/10.1016/j.bjm.2017.06.007
Fatima Z, Saleemi M, Zia M, Sultan T, Aslam M, Rehman RU, Chaudhary MF (2008) Antifungal activity of plant growth-promoting rhizobacteria isolates against Rhizoctonia solani in wheat. Afr J Biotechnol 8(2):219–225. https://doi.org/10.5897/AJB2009.000-9040
Pandey AK, Burlakoti RR, Rathore A, Nair RM (2020) Morphological and molecular characterization of Macrophomina phaseolina isolated from three legume crops and evaluation of mungbean genotypes for resistance to dry root rot. Crop Prot 127:104962. https://doi.org/10.1016/j.cropro.2019.104962
Javaid A, Afzal L, Shoaib A (2016) Biological control of charcoal rot of mungbean by Trichoderma harzianum and shoot dry biomass of Sisymbrium irio. Planta Daninha 2017(v35):e017165756. https://doi.org/10.1590/S0100-83582017350100075
Biswas K, Tarafdar A, Kumar R, Singhvi N, Ghosh P, Sharma M, Pabbi S, Shukla P (2020) Molecular analysis of disease-responsive genes revealing the resistance potential against Fusarium Wilt (Fusarium udum Butler) dependent on genotype variability in the leguminous crop Pigeonpea. Front Genet 11:862. https://doi.org/10.3389/fgene.2020.00862
Holt JG, Krieg NR, Peter PHA, Staley JT, Williams ST (1994) Bergeys manual of determinative bacteriology, 9th edn. William and Wilkins, Baltimore, p 559
Jhala YK, Vyas RV, Shelat HN, Patel HK, Patel HK, Patel KT (2014) Isolation and characterization of methane utilizing bacteria from wetland paddy ecosystem. World J Microbiol Biotechnol 30(6):1845–1860. https://doi.org/10.1007/s11274-014-1606-3
Sambrook J, Fritsch EF, Maniatis T (1989) Analysis and cloning of eukaryotic genomic DNA in Molecular cloning. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 9–19
Di Cello F, Bevivino A, Chiarini L, Fani R, Paffetti D, Tabacchioni S, Dalmastri C (1997) Biodiversity of a Burkholderia cepacia population isolated from the maize rhizosphere at different plant growth stages. Appl Environ Microbiol 63(11):4485–4493. https://doi.org/10.1128/aem.63.11.4485-4493.1997
Lee KD, Bai Y, Smith D, Han HS, Supanjani S (2005) Isolation of plant-growth-promoting endophytic bacteria from bean nodules. Res J Agric Biol Sci 1(3):232–236
Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, Chun J (2017) Introducing EzBioCloud: a taxonomically united database of 16S rRNA and whole genome assemblies. Int J Syst Evol Microbiol 67:1613–1617. https://doi.org/10.1099/ijsem.0.001755
Susilowati A, Wahyudi AT, Lestari Y, Suwanto A, Wiyono S (2011) Potential Pseudomonas isolated from Soybean rhizosphere as biocontrol against soilborne phytopathogenic fungi. HAYATI J Biosci 18(2):51–56. https://doi.org/10.4308/hjb.18.2.51
Milner JL, Stohl EA, Handelsman J (1996) Zwittermicin a resistance gene from Bacillus cereus. J Bacteriol 178(14):4266–4272. https://doi.org/10.1128/jb.178.14.4266-4272.1996
Padaria JC, Tarafdar A, Raipuria R, Lone SA, Gahlot P, Shakil NA, Kumar J (2016) Identification of phenazine-1-carboxylic acid gene (phc CD) from Bacillus pumilus MTCC7615 and its role in antagonism against Rhizoctonia solani. J Basic Microbiol 56:999–1008. https://doi.org/10.1002/jobm.201500574
Raffel SJ, Stabb EV, Milner JL, Handelsman J (1996) Genotypic and phenotypic analysis of zwitterrnicin A- producing strains of Bacillus cereus. Microbiology 142:3425–3436. https://doi.org/10.1099/13500872-142-12-3425
Bakker AW, Schippers B (1987) Microbial cyanides production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp. mediated plant growth stimulation. Soil Microbiol Biochem 19:451–457. https://doi.org/10.1016/0038-0717(87)90037-X
Uzair B, Kausar R, Bano SA, Fatima S, Badshah M, Habiba U, Fasim F (2018) Isolation and molecular characterization of a model antagonistic Pseudomonas aeruginosa divulging In Vitro plant growth promoting characteristics. Hindawi Bio Med Res Int 2018:1–7. https://doi.org/10.1155/2018/6147380
Durr SR (2014) Development of high-throughput methods for the detection of hydrogen cyanide-producing bacteria for the application in biocontrol. (Master’s thesis, University of Natural Resources and Life Sciences, Vienna). https://forschung.boku.ac.at/fis/suchen.hochschulschriften_info?sprache_in=en&menue_id_in=206&id_in=&hochschulschrift_id_in=11680
Raza W, Yang X, Wu H, Wang Y, Xu Y, Shen Q (2009) Isolation and characterisation of fusaricidin-type compound-producing strain of Paenibacillus polymyxa SQR-21 active against Fusarium oxysporum f.sp. nevium. Eur J Plant Pathol 125:471–483. https://doi.org/10.1007/s10658-009-9496-1
Naing KW, Anees M, Kim SJ, Nam Y, Kim YC, Kim KY (2014) Characterization of antifungal activity of Paenibacillus ehimensis KWN38 against soilborne phytopathogenic fungi belonging to various taxonomic groups. Ann Microbiol 64:55–63. https://doi.org/10.1007/s13213-013-0632-y
Schwyn B, Neilands JB (1987) Universal chemical assay for detection and determination of siderophores. Anal Biochem 160:47–56. https://doi.org/10.1016/0003-2697(87)90612-9
Smibert RM, Krieg NR (1994) Phenotypic characterization. In: Gerhardt P, Murray RGE, Wood WA, Krieg NR (eds) Methods for general and molecular bacteriology. American Society of Microbiology, Washington DC, pp 607–654
Sessitsch A, Reiter B, Berg G (2004) Endophytic bacterial communities of field grown potato plants and their plant growth promoting and antagonistic abilities. Can J Microbiol 50:239–249. https://doi.org/10.1139/w03-118
Lawrence RC, Fryer TF, Reiter B (1967) Rapid method for the quantitative estimation of microbial lipases. Nature 213:1264–1265. https://doi.org/10.1038/2131264a0
Salah A, Ibrahim A, El-diwany A (2007) Isolation and identification of new cellulases producing thermophilic bacteria from an Egyptian hot spring and some properties of the crude enzyme. Aust J Basic Appl Sci 1(4):473–478
Duncan DB (1955) Multiple range and multiple F tests. Biometrics 11:1–42. https://doi.org/10.2307/3001478
Basurto-Cadena MGL, Vazquez-Arista M, Garcıa-Jimenez J, Salcedo-Hernandez R, Bideshi DK, Barboza-Corona JE (2012) Isolation of a New Mexican strain of Bacillus subtilis with antifungal and antibacterial activities. Sci World J 2012:1–7. https://doi.org/10.1100/2012/384978
Anandaraj B, Leema RDA (2010) Studies on the influence of bio inoculants (Pseudomonas fluorescens, Rhizobium sp., Bacillus megaterium) in Green gram. J Biosci Technol 1(2):95–99
Dhole A, Shelat H, Vyas R, Jhala Y, Bhange M (2016) Endophytic occupation of legume root nodules by nifH-positive non-rhizobial bacteria, and their efficacy in the groundnut (Arachis hypogaea). Ann Microbiol 66:1397–1407. https://doi.org/10.1007/s13213-016-1227-1
Rajendran G, Sing F, Desai AJ, Archana G (2008) Enhanced growth and nodulation of pigeon pea by co-inoculation of Bacillus strains with Rhizobium spp. Bioresour Technol 99:4544–4550. https://doi.org/10.1016/j.biortech.2007.06.057
Stajkovic O, Meyer SD, Miličić B, Willems A, Delić D (2009) Isolation and characterization of endophytic non-rhizobial bacteria from root nodules of alfalfa (Medicago sativa L.). Botanica Serbica 33(1):107–113
Lin T, Zhao L, Yang Y, Guan Q, Gong M (2013) Potential of endophytic bacteria isolated from Sophora alopecuroides nodule in biological control against Verticillium wilt disease. Aust J Crop Sci 7(1):139–146
Pertot I, Puopolo G, Hosni T, Pedrotti L, Jourdan E, Ongena M (2013) Limited impact of abiotic stress on surfactin production in planta and on disease resistance induced by Bacillus amyloliquefaciens S499 in tomato and bean. FEMS Microbiol Ecol 86:505–519. https://doi.org/10.1111/1574-6941.12177
Andreolli M, Zapparoli G, Angelini E, Lucchetta G, Lampis S, Vallini G (2019) Pseudomonas protegens MP12: a plant growth-promoting endophytic bacterium with broad-spectrum antifungal activity against grapevine phytopathogens. Microbiol Res 219:123–131. https://doi.org/10.1016/j.micres.2018.11.003
Harman GE, Hayes CK, Lorito M, Broadway RM, Di Pietro A, Peterbaues C, Tronsmo A (1993) Chitinolytic enzymes of Trichoderma harzianum: purification of chitobiosidase and endochitinase. Phytopathology 83:313–318
Ordentlich A, Elad Y, Chet I (1988) The role of chitinase of Serratia marcescens in biocontrol of Sclerotium rolfsii. Phytopathology 78:48–88. https://doi.org/10.1016/0038-0717(87)90058-7
Shapira R, Ordentlich A, Chet I, Openheim AB (1989) Control of plant diseases by chitinase expressed from cloned DNA in Escherichia coli. Phytopathology 79:1246–1249. https://doi.org/10.1094/PHYTO-79-1246
Mauch F, Mauch-Mani B, Boller T (1988) Antifungal hydrolases in pea tissue. II. Inhibition of fungal growth by combinations of chitinase and L-1,3-glucanase. Plant Physiol 88:936–942. https://doi.org/10.1104/pp.88.3.936
Tseng S, Liu S, Yang H, Lo C, Peng K (2008) Proteomic study of biocontrol mechanisms of Trichoderma harzianum ETS 323 in response to Rhizoctonia solani. J Agric Food Chem 56:6914–6922. https://doi.org/10.1021/jf703626j
Ryu CM, Farag MA, Hu CH et al (2003) Bacterial volatiles promotegrowth in Arabidopsis. Proc Natl Acad Sci USA 100:4927–4932. https://doi.org/10.1073/pnas.0730845100
Laville J, Blumer C, Von Schroetter C, Gaia V, Défago G, Keel C, Haas D (1998) Characterization of the hcnABC gene cluster encoding hydrogen cyanide synthase and anaerobic regulation by ANR in the strictly aerobic biocontrol agent Pseudomonas fluorescens CHA0. J Bacteriol 180(12):3187–3196. https://doi.org/10.1128/JB.180.12.3187-3196.1998
Heydari S, Moghadam PR, Arab SM (2015) Hydrogen cyanide production ability by Pseudomonas Fluorescence bacteria and their inhibition potential on weed germination. www.tropentag.de/2008abstracts/full/676.pdf. Accessed 15 Oct 2018
Zhao L, Xu Y, Sun R, Deng Z, Yang W, Wei G (2011) Identification and characterization of the endophytic plant growth prompter Bacillus cereus strain MQ23 isolated from Sophora alopecuroides root nodules. Brazilian J Microbol 42:567–575. https://doi.org/10.1590/S1517-838220110002000022
Pandya M, Rajput M, Rajkumar S (2015) Exploring plant growth promoting potential of non rhizobial root nodules endophytes of V. radiata. Microbiology 84(1):110–119. https://doi.org/10.1134/S0026261715010105
Wedage WMM, Gunawardana D (2016) Rhizobial and non-Rhizobial nodulators of Pueraria phaseoloides. In: Proceedings of the 2016 international nitrogen initiative conference, Solutions to improve nitrogen use efficiency for the world, 4–8 December 2016, Melbourne, Australia. https://doi.org/10.13140/RG.2.2.25019.62245
Nandimath AP, Kharat KR, Gupta SG, Kharat AS (2016) Optimization of cellulase production for Bacillus sp. and Pseudomonas sp. soil isolates. Afr J Microbiol. Res 10(13):410–419. https://doi.org/10.5897/AJMR2016.7954
Sethi S, Datta A, Gupta BL, Gupta S (2013) Optimization of cellulase production from bacteria isolated from soil. ISRN Biotechnol 2013:1–7. https://doi.org/10.5402/2013/985685
Acknowledgements
Authors are thankful to Department of science and technology for providing financial assistance via INSPIRE Fellowship during the research at Anand Agricultural University, Anand.
Funding
The research was funded by the Department of science and technology via INSPIRE Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest associated with this publication.
Ethical Approval
This research did not involve any studies with human participants or animals (vertebrates) performed by any of the authors.
Consent to Participate
All Authors approve the article for the publication in Current Microbiology.
Consent for Publication
Authors are Consent for publication of the above work in Current Microbiology. We understand that the text and any figures published in the article will be freely available for public. The pictures and text may also appear on other websites or in print, may be translated into other languages or used for commercial purposes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dhole, A., Shelat, H. Non-Rhizobial Endophytes Associated with Nodules of Vigna radiata L. and Their Combined Activity with Rhizobium sp.. Curr Microbiol 79, 103 (2022). https://doi.org/10.1007/s00284-022-02792-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-02792-x