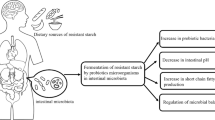
Abstract
The purpose of this study was to analyze the effects of instant dehydrated rice sticks (IDRS) which were substituted with resistant starch (RS) types 2 and 4 whose gut health function targets gut microbiota. IDRS are a type of rice noodles that were developed by two formulations. The first formulation had substitution of rice flour with 20% RS type 2 and 0.15% carboxymethyl cellulose (CMC) (RSc-2), and the second formulation had 25% RS type 4 and 0.15% CMC (RSc-4). RSc-2 and RSc-4 were investigated for gut health function by human fecal fermentation in a pH-controlled batch culture. The results of gut microbiota enumeration by fluorescent in situ hybridization confirmed that significantly (P < 0.05) higher numbers of bifidobacteria were obtained with RSc-2 (10.06 ± 0.09 log cells/mL) and RSc-4 (10.00 ± 0.06 log cells/mL) compared to the control (100% rice flour formula) at 24 h fermentation. Additionally, the prebiotic indexes of RSc-2 and RSc-4 were 3.8 and 2.8 -fold higher than that of the control at 24 h fermentation. The short-chained fatty acids, acetic, propionic and butyric acid were analyzed by gas chromatography–flame ionization detector. The butyric acids were significantly (P < 0.05) higher with RSc-2 (43.56 ± 0.01 mM) and RSc-4 (43.63 ± 0.07 mM) compared to the control at 24 h. Thus, RSc-2 and RSc-4 showed butyrogenic, bifidogenic and prebiotic potential to support gut health and could aid in prevention of colon cancer.



Similar content being viewed by others
Data Availability
All data generated or analyzed in this study are included in this published article and its supplementary files.
References
Nugent AP (2005) Health properties of resistant starch. Nutr Bull 30:27–54. https://doi.org/10.1111/j.1467-3010.2005.00481.x
Wu N, Tan B, Li S, Zhang M (2018) Quality characteristics of extruded brown rice noodles with different amylose contents. Food Sci Technol Res 24(2):311–319. https://doi.org/10.3136/fstr.24.311
Xioping Y, Darko KO, Huang Y, He C, Huansheng Y, He S, Li J, Hocher B, Yin Y (2017) Resistant starch regulates gut microbiota: structure, biochemistry and cell signalling: review. Cell Physiol Biochem 42:306–318. https://doi.org/10.1159/000477386
Correa J, Giannuzzi L, Weisstaub AR, Zuleta A, Ferrero C (2020) Chemically modified resistant starch in breadmaking: impact on bone, mineral metabolism and gut health of growing wistar rats. Int J Food Sci Technol 55:239–247. https://doi.org/10.1111/ijfs.14352
Rengadu D, Gerrano AS, Mellem JJ (2020) Prebiotic effect of resistant starch from Vigna unguiculata (L.) Walp. (cowpea) using an in vitro simulated digestion model. Int J Food Sci Technol 55(1):332–339. https://doi.org/10.1111/ijfs.14304
Bendiks ZA, Knudsen KEB, Keenan MJ, Marco ML (2020) Conserved and variable responses of the gut microbiome to resistant starch type 2. Nutr Res Rev 77:12–28. https://doi.org/10.1016/j.nutres.2020.02.009
Deehan EC, Yang C, Maria EP, Nguyen KN, Christopher CC, Lucila T, Zhengxiao Z, Jeffrey AB, Jens W (2020) Precision microbiome modulation with discrete dietary fiber structures directs short-chain fatty acid production. Cell Host Microbe 27(3):389-404.e6. https://doi.org/10.1016/j.chom.2020.01.006
Garg N, Singh A, Chaudhary DP (2017) Resistant starch: a potential impact on human health. Int J Curr Microbiol App Scie 6(5):2046–2057. https://doi.org/10.20546/ijcmas.2017.605.228
Bird AR, Conton MA, Christophersen CT, Topping DL (2010) Resistant starch, large bowel fermentation and a broader perspective of prebiotics and probiotics. Benef Microbes 1(4):423–431. https://doi.org/10.3920/BM2010.0041
Hung PV, Yamamori M, Morita N (2005) Formation of enzyme-resistant starch in bread as affected by high-amylose wheat flour substitutions. Cereal Chem 82(6):690–694. https://doi.org/10.1094/CC-82-0690
Dhital S, Surendra BK, Ashok KS (2010) Formation of resistant starch during processing and storage of instant noodles. Int J Food Prop 13(3):454–463. https://doi.org/10.1080/10942910802627091
Wichienchot S, Jaturpornpipat M, Rastall RA (2010) Oligosaccharides of pitaya (dragon fruit) flesh and their prebiotic properties. Food Chem 120(3):850–857. https://doi.org/10.1016/j.foodchem.2009.11.026
Pansai N, Chakree K, Yupanqui CT, Raungrut P, Yanyiam N, Wichienchot S (2020) Gut microbiota modulation and immune boosting properties of prebiotic dragon fruit oligosaccharides. Int J Food Sci Technol 55:55–64. https://doi.org/10.1111/ijfs.14230
Keenan MJ, Zhou J, Hegsted M, Pelkman C, Durham HA, Coulon DB, Martin RJ (2015) Role of resistant starch in improving gut health, adiposity, and insulin resistance. Adv Nutr 6(2):198–205. https://doi.org/10.3945/an.114.007419
Rinninella E, Cintoni M, Raoul P, Lopetuso LR, Scaldaferri F, Pulcini G, Miggiano GAD, Gasbarrini A, Mele MC (2019) Food components and dietary habits: keys for a healthy gut microbiota composition. Nutrients. 11(10):2393. https://doi.org/10.3390/nu11102393
Minekus M, Alminger M, Alvito P, Ballance S, Bohn T, Bourlieu C, Carrière F, Boutrou R, Corredig M, Dupont D, Dufour C, Egger L, Golding M, Karakaya S, Kirkhus B, Le Feunteun S, Lesmes U, MacIerzanka A, MacKie A, Marze S, McClements DJ, Ménard O, Recio I, Santos C, Singh RP, Vegarud GE, Wickham MSJ, Weitschies W, Brodkorb A (2014) A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct 5(6):1113–1124. https://doi.org/10.1039/c3fo60702j
Edwards CA (2003) Gums: dietary importance. In: Caballero B (ed) Encyclopedia of food sciences and nutrition, vol II. Academic Press, San Diego, pp 3007–3012
Lesmes U, Beards EJ, Gibson GR, Tuohy KM, Shimoni E (2008) Effects of resistant starch type III polymorphs on human colon microbiota and short chain fatty acids in human gut models. J Agric Food Chem 56:5415–5421. https://doi.org/10.1021/jf800284d
Owolabi IO, Dat-arun P, Yupanqui CT, Wichienchot S (2020) Gut microbiota metabolism of functional carbohydrates and phenolic compounds from soaked and germinated purple rice. J Funct Foods. https://doi.org/10.1016/j.jff.2020.103787
Plongbunjong V, Graidist P, Erik K, Knudsen B, Wichienchot S (2017) Starch-based carbohydrates display the bifidogenic and butyrogenic properties in pH-controlled faecal fermentation. Int J Food Sci Technol 2:2647–2653. https://doi.org/10.1111/ijfs.13553
Kedia G, Vázquez JA, Charalampopoulos D, Pandiella SS (2009) In vitro fermentation of oat bran obtained by debranning with a mixed culture of human fecal bacteria. Curr Microbiol 58(4):338–342. https://doi.org/10.1007/s00284-008-9335-1
Connolly ML, Lovegrove JA, Tuohy KM (2010) Konjac glucomannan hydrolysate beneficially modulates bacterial composition and activity within the faecal microbiota. J Funct Foods 2(3):219–224. https://doi.org/10.1016/j.jff.2010.05.001
Plongbunjong A, Graidist P, Knudsen KEB, Wichienchot S (2017) Starch-based carbohydrates display the bifidogenic and butyrogenic properties in pH-controlled faecal fermentation. Int J Food Sci and Tech 52:2647–2653. https://doi.org/10.1111/ijfs.13553
Palframan R, Gibson GR, Rastall RA (2003) Development of quantitative tool for the comparison of the prebiotic effect of dietary oligosaccharides. Lett Appl Microbiol 37:281–284. https://doi.org/10.1046/j.1472-765x.2003.01398.x
Plongbunjong V, Wichienchot S, Madla S, Bunyapipat P, Knudsen KEB, Graidist P (2019) Isomalto-oligosaccharide from rice and their potential use as pharma-nutraceuticals in prevention of colon cancer. Func Food Health Dis 9(6):371–383. https://doi.org/10.31989/ffhd.v9i6.598
DeMartino P, Cockburn DW (2020) Resistant starch: impact on the gut microbiome and health. Curr Opin Biotechnol 61:66–71. https://doi.org/10.1016/j.copbio.2019.10.008
Wang M, Wichienchot S, He X, Fu X, Huang Q (2019) In vitro colonic fermentation of dietary fibers: fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci Tech 88:1–9. https://doi.org/10.1016/j.tifs.2019.03.005
Koh A, Vadder DF, Datchary KP, Backhed F (2016) Review from dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165(6):1332–1345. https://doi.org/10.1016/j.cell.2016.05.041
Sánchez-Zapata E, Viuda-Martos M, Fernández-López J, Pérez-Alvarez JA (2015) Resistant starch as functional ingredient. In: Ramawat K, Mérillon JM (eds) Polysaccharides. Springer, Cham, pp 1911–1931
Ariestanti CA, Seechamnanturakit V, Harmayani E, Wichienchot S (2019) Optimization on production of konjac oligo-glucomannan and their effect on the gut microbiota. Food Sci Nutr 7(2):788–796. https://doi.org/10.1002/fsn3.927
Martínez I, Kim J, Duffy PR, Schlegel VL, Walter J (2010) Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS ONE 5:11. https://doi.org/10.1371/journal.pone.001504622
Swidsinski A, Ung V, Sydora BC, Loening-Baucke V, Doerffel Y, Verstraelen H, Fedorak RN (2009) Bacterial overgrowth and inflammation of small intestine after carboxymethylcellulose ingestion in genetically susceptible mice. Inflamm Bowel Dis 15(3):359–364. https://doi.org/10.1002/ibd.20763
Fuentes-Zaragoza E, Sanchez-Zapata E, Sendra E, Sayas E (2011) Resistant starch as prebiotic: a review. Starch/Stärke 63:406–415. https://doi.org/10.1002/star.201000099
Maier TV, Lucio M, Lee LH, VerBerkmoes NC, Brislawn CJ, Bernhardt J, Lamendella R, McDermott JE, Bergeron N, Heinzmann SS (2017) Impact of dietary resistant starch on the human gut microbiome, metaproteome, and metabolome. mBio 8:1–16. https://doi.org/10.1128/mBio.01343-17
Baxter TN, Schmidt AW, Vankataraman A, Kim KS, Waldron C, Schmidt TM (2019) Dynamics of human gut microbiota and short chain fatty acids in response to dietary interventions with three fermentable fibers. mBio 10:1–13. https://doi.org/10.1128/mBio.02566-18
Sindhu I, Chhibber S, Capalash N, Sharma P (2006) Production of cellulase-free xylanase from bacillus megaterium by solid state fermentation for biobleaching of pulp. Curr Microbiol 53(2):167–172. https://doi.org/10.1007/s00284-006-0051-4
Srikaeo K, Sangkhiaw J (2014) Effects of amylose and resistant starch on glycaemic index of rice noodles. LWT-Food Sci Technol 59(2):1129–1135. https://doi.org/10.1016/j.lwt.2014.06.012
Hald S, Schioldan AG, Moore ME, Dige A, Lærke HN, Agnholt J, Knudsen KEB, Hermansen K, Marco ML, Gregersen S, Dahlerup JF (2016) Effects of arabinoxylan and resistant starch on intestinal microbiota and short-chain fatty acids in subjects with metabolic syndrome: a randomised crossover study. PLoS ONE 11(7):1–18. https://doi.org/10.1371/journal.pone.0159223
Haenen D, Zhang J, Silva SC, Bosch G, Meer IM, Arkel J, Borne JJGC, Gutiérrez PO, Smidt H, Kemp B, Müller M, Hooiveld GJEJ (2013) A diet high in resistant starch modulates microbiota composition, SCFA concentrations, and gene expression in pig intestine. J Nutr 143(3):274–283. https://doi.org/10.3945/jn.112.169672
Erickson JM, Carlson JL, Stewart ML, Slavin JL (2018) Fermentability of novel type-4 resistant starches in in vitro system. Foods 7(18):1–14. https://doi.org/10.3390/foods7020018
Leu LRK, Hu Y, Brown IL, Young GP (2009) Effect of high amylose maize starches on colonic fermentation and apoptotic response to DNA-damage in the colon of rats. Nutr Metab 6:11. https://doi.org/10.1186/1743-7075-6-11
Kim KN, Yao Y, Ju SY (2019) Short chain fatty acids and fecal microbiota abundance in humans with obesity: a systematic review and meta-analysis. Nutrients. https://doi.org/10.3390/nu11102512
Venegas DP, Fuente MKD, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, Hermoso MA (2019) Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol 10:277. https://doi.org/10.3389/fimmu.2019.00277
Acknowledgements
The authors gratefully acknowledge financial support to the study from Thailand Education Hub for Southern Region of ASEAN Countries (scholarship no. TEH-AC 008/2018), research grant support from the Institute of Food Research Innovation, Prince of Songkla University and Thai Preserved Food Factory Company Limited, Nakhon Pathom, Thailand (Grant No. 2561/016) and Dr. Seppo Karrila for the language editing and profreading of this journal.
Funding
Thailand Education Hub for Southern Region of ASEAN Countries (Scholarship No. TEH-AC 008/2018)—“Institute of Food Research Innovation, Prince of Songkla University and Thai Preserved Food Factory Company Limited, Nakhon Pathom, Thailand (Grant No. 2561/016)”.
Author information
Authors and Affiliations
Contributions
NA and SW conceived the study idea. NA carried out the experiments and wrote the manuscript with support from SW who supervised this study. PS contributed to analyzing quality of the product. NA and SW contributed to final version of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare not having any conflict of interest.
Informed Consent
The study performed had received ethical clearance to obtain feces samples from donors. The ethical permission was approved by the EC committee of the institute (Prince of Songkla University, Thailand) with an expedited process since sampling the feces was not harmful to the donors. Consents were freely provided by all donors and the principal investigator has a good clinical practice (GCP) certificate.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
284_2021_2564_MOESM1_ESM.docx
Supplementary file1 Fig. S1 Representative images of the FISH experiment of instant dehydrated rice sticks without substitution of RS (control) at 24 h of fecal batch fermentation: (a) bifidobacteria, (b) lactobacilli, (c) clostridia, (d) bacteroides, and (e) eubacteria. Fig. S2 Representative images of the FISH experiment of instant dehydrated rice sticks with 25% RS type 4 and 0.15% CMC (RSc-4) at 24 h of fecal batch fermentation: (a) bifidobacteria, (b) lactobacilli, (c) clostridia, (d) bacteroides, and (e) eubacteria (DOCX 4700 kb)
Rights and permissions
About this article
Cite this article
Alfilasari, N., Sirivongpaisal, P. & Wichienchot, S. Gut Health Function of Instant Dehydrated Rice Sticks Substituted with Resistant Starch Types 2 and 4. Curr Microbiol 78, 3010–3019 (2021). https://doi.org/10.1007/s00284-021-02564-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-021-02564-z