Abstract
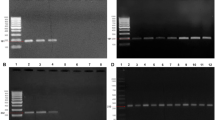
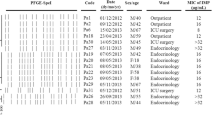
Pseudomonas aeruginosa is associated with chronic and progressive lung disease and is closely related to increased morbidity and mortality in cystic fibrosis (CF) patients. Hypermutable (HPM) P. aeruginosa isolates have been described in these patients and are usually associated with antibiotic resistance. This study aimed to investigate the occurrence of carbapenem resistance and hypermutable phenotype in 179 P. aeruginosa isolates from 8 chronically CF patients assisted at two reference centers in Rio de Janeiro, Brazil. Using disk diffusion test, non-susceptible (NS) rates higher than 40% were observed for imipenem, amikacin, and gentamicin. A total of 79 isolates (44.1%), 71 (39.6%), and 8 (4.4%) were classified as carbapenem-resistant (CR resistance to at least one carbapenem), multidrug-resistant (MDR), and extensively drug-resistant (XDR), respectively. Minimal inhibitory concentration was determined for 79 CR P. aeruginosa and showed the following variations: 4 and 128 μg/mL to imipenem, 4 and 64 µg/mL to meropenem, and 4 and ≥ 32 µg/mL to doripenem. We have found only four (2.23%) HPM isolates from 4 patients. Analyzing the genetic relationship among the HPM isolates, 3 pulsed-field gel electrophoresis/pulsotypes (D, M, and J) were observed. Only M pulsotype was recovered from two patients in different years. Polymerase chain reaction screening for blaGES, blaIMP, blaKPC, blaNDM, blaOXA-48, blaSPM, and blaVIM genes was performed for all CR isolates and none of them were positive. Our results demonstrate a high occurrence of CR and MDR P. aeruginosa of CF patients follow-up in both centers studied, while the presence of HPM is still unusual.
Similar content being viewed by others
Availability of Data and Materials
All data generated or analyzed during this study are included in this published article.
References
Bhagirath AY, Li Y, Somayajula D, Dadashi M, Badr S, Duan K (2016) Cystic fibrosis lung environment and Pseudomonas aeruginosa infection. BMC Pulm Med 16:1–22. https://doi.org/10.1186/s12890-016-0339-5
Finnan S, Morrissey JP, Gara FO, Boyd EF (2004) Genome diversity of Pseudomonas aeruginosa isolates from cystic fibrosis patients and the hospital environment. J Clin Microbiol 42:5783–5792. https://doi.org/10.1128/JCM.42.12.5783-5792.2004
Mustafa M, Chalhoub H, Denis O, Deplano A, Vergison A, Rodriguez-villalobos H, Tunney MM, Elborn JS, Kahl BC, Traore H, Vanderbist F, Tulkens PM (2016) Antimicrobial susceptibility of Pseudomonas aeruginosa isolated from cystic fibrosis patients in Northern Europe. Antimicrob Agents Chemother 60:6735–6741. https://doi.org/10.1128/AAC.01046-16
Maciá MD, Blanquer D, Togores B, Sauleda J, Perez JL, Oliver A (2005) Hypermutation is a key factor in development of multiple-antimicrobial resistance in Pseudomonas aeruginosa strains causing chronic lung infections. Antimicrob Agents Chemother 49:3382–3386. https://doi.org/10.1128/AAC.49.8.3382-3386.2005
Feliziani S, Luján AM, Moyano AJ, Sola C, Bocco JL, Montanaro P, Canigia LF, Argaraña CE, Smania AM (2010) Mucoidy, quorum sensing, mismatch repair and antibiotic resistance in Pseudomonas aeruginosa from cystic fibrosis chronic airways infections. PLoS ONE 5:1–12. https://doi.org/10.1371/journal.pone.0012669
Lutz L, Leão RS, Ferreira AG, Pereira DC, Raupp C, Pitt T, Marques EA, Barth AL (2013) Hypermutable Pseudomonas aeruginosa in cystic fibrosis patients from two Brazilian cities. J Clin Microbiol 51:927–930. https://doi.org/10.1128/JCM.02638-12
Logan LK, Gandra S, Mandal S, Klein EY, Levinson J, Weinstein RA, Laxminarayan R (2017) Multidrug- and carbapenem-resistant Pseudomonas aeruginosa in children, United States, 1999–2012. J Pediatr Infect Dis Soc 6:352–359. https://doi.org/10.1093/jpids/piw064
Rutter WC, Burgess DR, Burgess DS (2016) Increasing incidence of multidrug resistance among cystic fibrosis respiratory bacterial isolates. Microb Drug Resist. https://doi.org/10.1089/mdr.2016.0048
Pedersen SS, Koch C, Hoibyt N, Roscndalj K (1986) An epidemic spread of multiresistant Pseudomonas aeruginosa in a cystic fibrosis centre. J Antimicrob Chemother 17:505–516. https://doi.org/10.1093/jac/17.4.505
Romling U, Wingender J, Muller H, Tummler B (1994) A major Pseudomonas aeruginosa clone common to patients and aquatic habitats. Appl Environ Microbiol 60:1734–1738
Cheng K, Smyth RL, Govan JR, Doherty C, Winstanley C, Denning N, Heaf DP, van Saene H, Hart CA (1996) Spread of beta-lactam-resistant Pseudomonas aeruginosa in a cystic fibrosis clinic. Lancet 348:639–642. https://doi.org/10.1016/S0140-6736(96)05169-0
Jones AM, Govan JR, Doherty CJ, Dodd ME, Isalska BJ, Nigel Stanbridge T, Kevin Webb A (2001) Spread of a multiresistant strain of Pseudomonas aeruginosa in an adult cystic fibrosis clinic. Lancet 358:557–558. https://doi.org/10.1016/s0140-6736(01)05714-2
Armstrong D, Bell S, Robinson M, Bye P, Rose B, Harbour C, Lee C, Service H, Nissen M, Syrmis M, Wainwright C (2003) Evidence for spread of a clonal strain of Pseudomonas aeruginosa among cystic fibrosis clinics. J Clin Microbiol 41:2266–2267. https://doi.org/10.1128/jcm.41.5.2266-2267.2003
Scott FW, Pitt TL (2004) Identification and characterization of transmissible Pseudomonas aeruginosa strains in cystic fibrosis patients in England and Wales. J Med Microbiol 53:609–615. https://doi.org/10.1099/jmm.0.45620-0
López-Causape C, Rojo-Molinero E, Mulet X, Cabot G, Moya B, Figuerola J, Togores B, Perez JL, Oliver A (2013) Clonal dissemination, emergence of mutator lineages and antibiotic resistance evolution in Pseudomonas aeruginosa cystic fibrosis chronic lung infection. PLoS ONE 8:1–8. https://doi.org/10.1371/journal.pone.0071001
Somayaji R, Lam JC, Surette MG, Waddell B, Rabin HR, Sibley CD, Purighalla S, Parkins MD (2017) Long-term clinical outcomes of ‘Prairie Epidemic Strain’ Pseudomonas aeruginosa infection in adults with cystic fibrosis. Thorax 72:333–339. https://doi.org/10.1136/thoraxjnl-2015-208083
Gniadek TJ, Carroll KC, Simner PJ (2016) Carbapenem-resistant non-glucose-fermenting Gram-negative Bacilli: the missing piece to the puzzle. J Clin Microbiol 54:1700–1710. https://doi.org/10.1128/JCM.03264-15
Poirel L, Pitout JD, Nordmann P (2007) Carbapenemases: molecular diversity and clinical consequences. Future Microbiol 2:501–512. https://doi.org/10.2217/17460913.2.5.501
Dortet L, Poirel L, Nordmann P (2012) Rapid identification of carbapenemase types in Enterobacteriaceae and Pseudomonas spp. by using a biochemical test. Antimicrob Agents Chemother 56:6437–6440. https://doi.org/10.1128/AAC.01395-12
Li Y, Zhang X, Wang C, Hu Y, Niu X, Pei D, He Z, Bi Y (2014) Characterization by phenotypic and genotypic methods of metallo-B-lactamase-producing Pseudomonas aeruginosa isolated from patients with cystic fibrosis. Mol Med Rep 11:494–498. https://doi.org/10.3892/mmr.2014.2685
Gales AC, Menezes LC, Silbert S, Sader HS (2003) Dissemination in distinct Brazilian regions of an epidemic carbapenem-resistant Pseudomonas aeruginosa producing SPM metallo-β-lactamase. J Antimicrob Chemother 52:699–702. https://doi.org/10.1093/jac/dkg416
Zavascki AP, Gaspareto PB, Martins AF, Gonçalves AL, Barth AL (2005) Outbreak of carbapenem-resistant Pseudomonas aeruginosa producing SPM-1 metallo-B-lactamase in a teaching hospital in southern Brazil. J Antimicrob Chemother 56:1148–1151. https://doi.org/10.1093/jac/dki390
Franco MR, Caiaffa-filho HH, Burattini MN, Rossi F (2010) Metallo-beta-lactamases among imipenem-resistant Pseudomonas aeruginosa in a Brazilian university hospital. Clinics 65:825–829. https://doi.org/10.1590/s1807-59322010000900002
Jácome PR, Alves LR, Cabral AB, Lopes AC, Maciel MA (2012) First report of KPC-producing Pseudomonas aeruginosa in Brazil. Antimicrob Agents Chemother 56:4990. https://doi.org/10.1128/AAC.00699-12
de Oliveira Santos IC, Albano RM, Asensi MD, D’Alincourt Carvalho-Assef AP (2018) Draft genome sequence of KPC-2-producing Pseudomonas aeruginosa recovered from a bloodstream infection sample in Brazil. J Glob Antimicrob Resist 15:99–100. https://doi.org/10.1016/j.jgar.2018.08.021
Galetti R, Andrade LN, Varani AM, Darini ALC (2019) SPM-1-producing Pseudomonas aeruginosa ST277 carries a chromosomal pack of acquired resistance genes: an example of high-risk clone associated with intrinsic resistome. J Glob Antimicrob Resist 16:183–186. https://doi.org/10.1016/j.jgar.2018.12.009
Ferreira AG, Leão RS, Carvalho-Assef DA, da Silva EA, Firmida MC, Folescu TW, Paixão VA, Santana MA, de Abreu e Silva FA, Barth AL, Marques EA (2015) Low-level resistance and clonal diversity of Pseudomonas aeruginosa among chronically colonized cystic fibrosis patients. APMIS 123:1061–1068. https://doi.org/10.1111/apm.12463
Lee B, Haagensen JA, Ciofu O, Andersen JB, Høiby N, Molin S (2005) Heterogeneity of biofilms formed by nonmucoid Pseudomonas aeruginosa isolates from patients with cystic fibrosis. J Clin Microbiol 43:5247–5255. https://doi.org/10.1128/JCM.43.10.5247-5255.2005
Clinical and Laboratory Standard Institute (CLSI) (2018) M100 standards, performance testing, antimicrobial susceptibility, 28th edn. Clinical and Laboratory Standard Institute (CLSI), Wayne, PA
Clinical and Laboratory Standard Institute (CLSI) (2018) M02 performance standards for antimicrobial disk susceptibility tests, 13th edn. Clinical and Laboratory Standard Institute (CLSI), Wayne, PA
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. https://doi.org/10.1111/j.1469-0691.2011.03570.x
Clinical and Laboratory Standard Institute (CLSI) (2018) M07 methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 28th edn. Clinical and Laboratory Standard Institute (CLSI), Wayne, PA
Oliver A, Cantón R, Campo P, Baquero F, Blásquez J (2016) High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Am Assoc Adv Sci 288:1251–1253. https://doi.org/10.1126/science.288.5469.1251
Tsakris A, Pournaras S, Woodford N, Palepou MF, Babini GS, Douboyas J, Livermore DM (2000) Outbreak of infections caused by Pseudomonas aeruginosa producing VIM-1 carbapenemase in Greece. J Clin Microbiol 38:1290–1292
Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, Alberti S, Bush K, Tenover FC (2001) Novel carbapenem-hydrolysing B-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 45:1151–1161. https://doi.org/10.1128/AAC.45.4.1151-1161.2001
Shibata N, Doi Y, Yamane K, Yagi T, Kurokawa H, Shibayama K, Kato H, Kai K, Arakawa Y (2003) PCR typing of genetic determinants for metallo-B-lactamases and integrases carried by Gram-negative bacteria isolated in Japan, with focus on the class 3 integron. J Clin Microbiol 41:5407–5413. https://doi.org/10.1128/jcm.41.12.5407-5413.2003
Lee K, Park AJ, Kim MY, Lee HJ, Cho J, Kang JO, Yong D, Chong Y (2009) Metallo-B-lactamase producing Pseudomonas spp. in Korea: high prevalence of isolates with VIM-2 type and emergence of isolates with IMP-1 type. Yonsei Med J 50:335–339. https://doi.org/10.3349/ymj.2009.50.3.335
Monteiro J, Widen RH, Pignatari AC, Kubasek C, Silbert S (2012) Rapid detection of carbapenemase genes by multiplex real-time PCR. J Antimicrob Chemother 67:906–909. https://doi.org/10.1093/jac/dkr563
Sader HS, Pignatari AC, Leme IL, Burattini MN, Tancresi R, Hollis RJ, Jones RN (1993) Epidemiologic typing of multiply drug-resistant Pseudomonas aeruginosa isolated from an outbreak in an intensive care unit. Diagn Microbiol Infect Dis 17:13–18. https://doi.org/10.1016/0732-8893(93)90063-d
Loureiro MM, De Moraes BA, Mendonça VL, Quadra MR, Pinheiro GS (2002) Pseudomonas aeruginosa: study of antibiotic resistance and molecular typing in hospital infection cases in a neonatal intensive care unit from Rio de Janeiro City, Brazil. Memórias do Instituto Oswaldo Cruz 97:387–394. https://doi.org/10.1590/s0074-02762002000300020
Parkins MD, Glezerson BA, Sibley CD, Sibley KA, Duong J, Purighalla S, Mody CH, Workentine ML, Storey DG, Surette MG, Rabin HR (2014) Twenty-five-year outbreak of Pseudomonas aeruginosa infecting individuals with cystic fibrosis: identification of the Prairie epidemic strain. J Clin Microbiol 52:1127–1135. https://doi.org/10.1128/JCM.03218-13
Waters VJ, Kidd TJ, Canton R, Ekkelenkamp MB, Johansen HK, LiPuma JJ, Bell SC, Elborn JS, Flume PA, VanDevanter DR, Gilligan P (2013) Reconciling antimicrobial susceptibility testing and clinical response in antimicrobial treatment of chronic cystic fibrosis lung infections. Infect Dis Soc Am. https://doi.org/10.1093/cid/ciz364
Salsgiver EL, Fink AK, Knapp EA, Lipuma JJ, Olivier KN, Marshall BC, Saiman L (2016) Changing epidemiology of the respiratory bacteriology of patients with cystic fibrosis. Chest 149:390–400. https://doi.org/10.1378/chest.15-0676
Damas C, Amorim A, Gomes I (2008) Fibrose quística: revisão. Rev Port Pneumol 14:89–112
Gaspar MC, Couet W, Olivier JC, Pais AA, Sousa JJ (2013) Pseudomonas aeruginosa infection in cystic fibrosis lung disease and new perspectives of treatment: a review. Eur J Clin Microbiol Infect Dis 32:1231–1252. https://doi.org/10.1007/s10096-013-1876-y
Pournajaf A, Razavi S, Irajian G, Ardebili A, Erfani Y, Solgi S, Yaghoubi S, Rasaeian A, Yahyapour Y, Kafshgari R, Shoja S, Rajabnia R (2018) Integron types, antimicrobial resistance genes, virulence gene profile, alginate production and biofilm formation in Iranian cystic fibrosis Pseudomonas aeruginosa isolates. Le Infezione in Medicina 3:226–236
Paixão VA, Barros TF, Mota CMC, Moreira TF, Santana MA, Reis JN (2010) Prevalence and antimicrobial susceptibility of respiratory pathogens in patients with cystic fibrosis. Braz J Infect Dis 14:406–409
Perez R, Limberger MF, Costi R, Dias G, Barth AL (2014) Evaluation of tests to predict metallo-B-lactamase in cystic fibrosis (CF) and non-(CF) Pseudomonas. Braz J Microbiol 45:835–839. https://doi.org/10.1590/s1517-83822014000300011
Leão RS, Pereira RH, Folescu TW, Albano RM, Santos EA, Junior LG, Marques EA (2011) KPC-2 carbapenemase-producing Klebsiella pneumoniae isolates from patients with cystic fibrosis. J Cyst Fibros 10:140–142. https://doi.org/10.1016/j.jcf.2010.12.003
Alves DP, Carvalho-Assef APD’A, Conceição-Neto OC, Aires CAM, Albano RM, Folescu TW, Ornelas SCS, Leão RS, Marques EA (2018) Enterobacter cloacae harbouring bla KPC-2 and qnrB-1 isolated from a cystic fibrosis patient: a case report. New Microbes New Infect 25:49–51. https://doi.org/10.1016/j.nmni.2018.06.010
Ciofu O, Riis B, Pressler T, Enghusen H, Høiby N, Poulsen HE (2005) Occurrence of hypermutable Pseudomonas aeruginosa in cystic fibrosis patients is associated with the oxidative stress caused by chronic lung inflammation. Antimicrob Agents Chemother 49:2276–2282. https://doi.org/10.1128/AAC.49.6.2276-2282.2005
Oliver A, Baquero F, Blázquez J (2002) The mismatch repair system (mutS, mutL and uvrD genes) in Pseudomonas aeruginosa: molecular characterization of naturally occurring mutants. Mol Microbiol 43:1641–1650. https://doi.org/10.1046/j.1365-2958.2002.02855.x
Maciá MD, Borrell N, Pérez JL, Oliver A (2004) Detection and susceptibility testing of hypermutable Pseudomonas aeruginosa strains with the Etest and disk diffusion detection and susceptibility testing of hypermutable Pseudomonas aeruginosa strains with the Etest and disk diffusion. Antimicrob Agents Chemother 48:2665–2672. https://doi.org/10.1128/AAC.48.7.2665-2672.2004
Mena A, Smith E, Burns JL, Speert DP, Moskowitz SM, Perez JL, Oliver A (2008) Genetic adaptation of Pseudomonas aeruginosa to the airways of cystic fibrosis patients is catalysed by hypermutation. J Bacteriol 190:7910–7917. https://doi.org/10.1128/JB.01147-08
Acknowledgements
This work was funded by INPRA—Instituto Nacional de Pesquisa em Resistência Antimicrobiana—Brazil, CNPq 465718/2014-0, FAPERGS 17/2551-0000514-7. This study was also partially supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasil (CAPES), Finance Code 001.
Funding
This work was funded by INPRA—Instituto Nacional de Pesquisa em Resistência Antimicrobiana—Brazil, CNPq 465718/2014–0, FAPERGS 17/2551-0000514-7. This study was also partially supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasil (CAPES), Finance Code 001.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Study conception and design were performed by EAM and RSL. Most of material preparation, data collection, and phenotypic and molecular analysis were performed by MMA. Material preparation, data collection, and phenotypic analysis were performed by MMA and MTF. Molecular analysis by PFGE was performed by EAM, RSL, MMA, and APD´ACA. Clinical data collection and analysis were performed by TWF and MCF. The first draft of the manuscript was written by MMA, EAM, and RSL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical Approval
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee (CAAE: 79547616.1.0000.5259), and the approval was waived by the local Ethics Committee of Universidade do Estado do Rio de Janeiro, in view of the retrospective nature of the study and all the procedures were performed with samples stored in a bacteriological collection.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Almeida, M.M., Freitas, M.T., Folescu, T.W. et al. Carbapenem-Resistant Pseudomonas aeruginosa in Chronic Lung Infection: Current Resistance Profile and Hypermutability in Patients with Cystic Fibrosis. Curr Microbiol 78, 696–704 (2021). https://doi.org/10.1007/s00284-020-02337-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02337-0