Abstract
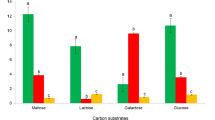
Production of carbon dioxide, as one of the ultimate products of fungal metabolism, can be used to quantify and measure their metabolic rate under different conditions, thus aiding in finding the optimal substrate and environment for cultivation of wood-destroying fungi. This study is focused on species Pleurotus ostreatus and Ganoderma lucidum,. These species are also cultivated for mycorestoration as well as their medicinal and nutritional value. To quantify their metabolical rate on various substrates (agar medium, wood chips, rye straw), multiple custom-built airtight chambers were equipped with CO2 probes (GMP 343, Vaisala, Finland) to measure the production of carbon dioxide. The highest values were measured during the primordial production on rye straw substrate, with the average values of 1.09 g CO2 kg−1 (substrate) h−1. These values varied significantly between various substrates, fungal species and development stages.






Similar content being viewed by others
References
Chang ST, Wasser SP (2012) The role of culinary-medicinal mushrooms on human welfare with a pyramid model for human health. Int J Med Mushrooms 14(2):93–134
Pauli G (1996) Breakthroughs: what business can offer society. Epsilon Press, Surrey, U.K.
Chang ST (2006) Development of the culinary-medicinal mushrooms industry in China: past, present, and future. Int J Med Mushrooms 8:1–12
Pavlík M, Byandusya P (2016) Effectiveness of the oyster mushroom growing on the locally available substrates in rural regions of Africa and Europe. In: Science and cultivation of edible and medicinal fungi: proceedings of the IXXth ISMS/Amsterdam/the Netherlands/30 May–2 June 2016, p 221–225
Buswell JA, Chang ST (1994) Biomass and extracellular hydrolytic enzyme production by six mushroom species grown on soybean waste. Biotechnol Lett 16:1317–1322
Stamets P (2005) Mycelium running. Ten Speed Press, Berkeley, CA
Hraško M, Pavlík M, Halaj D (2014) Profitability assessment of the oyster mushroom on chosen wood species in conditions of the forestry. In: GeoConference on water resources: conference proceedings. Volume II.—Sofia: STEF92 Technology: 427–434
Chang ST, Wasser SP (2017) The cultivation and environmental impact of mushrooms. Oxf Res Encycl Environ Sci. https://doi.org/10.1093/acrefore/9780199389414.013.231
Lindahl B, Boberg J (2007) Distribution and function of litter basidiomycetes in coniferous forests. In: Boddy L, Frankland J, van West P (eds) Ecology of saprotrophic basidiomycetes. Elsevier Ltd., London, pp 183–196
Purnomo AS, Mori T, Putra SR, Kondo R (2013) Biotransformation of heptachlor and heptachlor epoxide by white-rot fungus Pleurotus ostreatus. Int Biodeterior Biodegrad 82:40–44
Rhodes CJ (2014) Mycoremediation (bioremediation with fungi): growing mushrooms to clean the earth. Chem Spec Bioavailab 26(3):196–198
Pavlík M, Krupová D, Ungvarská-Maľučká L, Pavlik M jr., Rajtar M (2017) Preliminary mycoremediation evaluation of waste ash from thermal power plant utilising the oyster mushroom. In: 17th international multidisciplinary scientific geoconference SGEM 2017, vol 17, issue 43:147–154
Wasser SP (2014) Medicinal mushroom science: current perspectives, advances, evidences, and challenges. Biomed J 35(6):516–528
Herrmann S, Bauhus J (2012) Effects of moisture, temperature and decomposition stage on respirational carbon loss from coarse woody debris (CWD) of important European tree pecies. Scand J For Res 28:346–357
Kahl T, Baber K, Otto P, Wirthl Ch, Bauhus J (2015) Drivers of CO2 emission rates from dead wood logs of 13 tree species in the initial decomposition phase. Forests 6:2484–2504. https://doi.org/10.3390/f6072484
Stamets P (2000) Growing gourmet and medicinal mushrooms. Ten Speed Press, Berkeley, CA
Golian M, Andrejiová A, Mezeyová I, Hegedusova A (2015) Design of oyster (Pleurotus ostreatus) production unit taking into account its agrotechnic of growing and quality and quantity of its production. J Microbiol Biotech Food Sci 4(3):48–51
Livingstone GP, Hutchinson GL (1995) Enclosure–based measurement of trace gas exchange: aplications and sources of error. In: Matsos PA, Harriss RC (eds) Biogenetic trace gases: measuring emissions from soil and water. Blackwell Science, Cambridge, pp 14–50
Drewitt GB, Black T, Nesic Z, Humphreys ER, Jork EM, Swanson R, Morgenstern K (2002) Measuring forest floor CO2 fluxes in a Douglas-fir forest. Agric For Meteorol 110(4):299–317
Linder S, Troeng E (1981) The seasonal variation in stem and coarse root respiration of a 20-year-old Scots pine (Pinus sylvestris L.). In: Tranquillini W (ed) Dick enwachstum der Bäume, Mitt. Forstl., vol 142. Bundesversuchsanst, Wien, pp 125–140
Percival DB, Walden AT (2000) Wavelet methods for time series analysis. Cambridge series in statistical and probabilistic mathematics. Cambridge University Press, Cambridge
Torrence C, Compo GP (1998) A practical guide to wavelet analysis. Bull Am Met Soc 79:61–78
Kulshreshtha S, Mathur N, Bhatnagar P (2013) Mycoremediation of paper, pulp, and cardboard industrial wastes and pollutants. In: Goltapeh EM, Danesh YR, Varma A (eds) Fungi as bioremediators: soil biology. Springer, Berlin, Berlin, pp 77–116
Farber JN, Harris LJ, Parish ME, Beuchat LR, Suslow TV, Gorney JR, Garrett EH, Busta FF (2003) Microbiological safety of controlled and modified atmosphere packaging of fresh and fresh-cut produce. Compr Rev Food Sci Food Saf 2:142–160
Fonseca SC, Oliveira AR, Brecht JK (2002) Modeling respiration rate of fresh fruits and vegetables for modified atmosphere packages: areview. J Food Eng 52:99–119
Heinemeyer A, Ineson P, Ostle N, Fitter AH (2006) Respiration of the external mycelium in the arbuscular mycorrhizal symbiosis shows strong dependence on recent photosynthates and acclimation to temperature. New Phytol 171:159–170
Rinne-Garmston KT, Peltoniemi K, Chen J, Peltoniemi M (2019) Carbon flux from decomposing wood and its dependency on temperature, wood N 2 fixation rate, moisture and fungal composition in a Norway spruce forest. Glob Change Biol 25:1852–1867. https://doi.org/10.1111/gcb.14594
Barba J, Cueva A, Bahn M et al (2017) Comparing ecosystem and soil respiration: review and key challenges of tower-based and soil measurements. Agric For Meteorol. https://doi.org/10.1016/j.agrformet.2017.10.028
Kahl T, Baber K, Otto P et al (2015) Drivers of CO2 emission rates from dead wood logs of 13 tree species in the initial decomposition phase. Forests 6:2484–2504. https://doi.org/10.3390/f6072484
Gitarskiy ML, Zamolodchikov DG, Mukhin VA, Grabar VA, Diyarova DK, Ivashenko AI (2017) Carbon fluxes from coarse woody debris in southern Taiga forests of the Valdai upland. Rus J Ecol 48:539–544. https://doi.org/10.1134/S1067413617060030
Trocha LK, Chen W, Dabert M, Eissenstat DM (2016) Linking the respiration of fungal sporocarps with their nitrogen concentration: variation among species, tissues and guilds. Funct Ecol 30:1756–1768. https://doi.org/10.1111/1365-2435.12688
Colpaert JV, Van Laere A (1996) A comparison of the extracellular enzyme activities of two ectomycorrhizal and a leaf-saprotrophic basidiomycete colonizing beech leaf litter. New Phytol 133:133–141
Sudheer S, Taha Z, Manickam S et al (2018) Development of antler-type fruiting bodies of Ganoderma lucidum and determination of its biochemical properties. Fungal Biol 122:293–301. https://doi.org/10.1016/j.funbio.2018.01.007
Loyd A, Loyd AL, Held BW et al (2018) Elucidating wood decomposition by four species of Ganoderma from the United States elucidating wood decomposition by four species of Ganoderma from the United States. Fungal Biol 122:254–263. https://doi.org/10.1016/j.funbio.2018.01.006
Belletini MB, Fiorda FA, Maieves HA, Teixeira GL, Avila S et al (2019) Factors affecting mushroom Pleurotus spp. Saudi J Biol Sci 26:633–646
Rock J, Badeck FW, Harmon MF (2008) Estimating decomposition rate constants for European tree species from literature sources. Eur J For Res. https://doi.org/10.1007/s10342-008-0206-x
Weedon JT, Cornwell K, Cornekissen JHC, Zanne AE, Wirth C, Coomes DA (2009) Global meta-analysis of wood decomposition rates: a role for trait variation among tree species ? Ecol Lett 12:45–56. https://doi.org/10.1111/j.1461-0248.2008.01259.x
Malcolm GM, Gutierrez JCL, Koide RT, Eisenstat DM (2008) Acclimation to temperature and temperature sensitivity of metabolism by ectomycorrhizal fungi. Glob Change Biol 14:1169–1180. https://doi.org/10.1111/j.1365-2486.2008.01555.x
Muller MM, Hamberg L, Kuuskeri J et al (2015) Respiration rate determinations suggest Heterobasidion parviporum subpopulations have potential to adapt to global warming. For Pathol 45:515–524. https://doi.org/10.1111/efp.12203
Robinson CH (2001) Cold adaptation in Arctic and Antarctic fungi. New Phytol 151:341–353
Lilleskov EA (2017) How does temperature affect forest “fungus breath”? Diurnal non-exponential temperature-respiration relationship, and possible longer-term acclimation in fungal sporocarps. Fungal Ecol 27:24–35. https://doi.org/10.1016/j.funeco.2017.02.001
Aghajani H, Bari E, Bahmani M, Humar M (2018) Influence of relative humidity and temperature on cultivation of Pleurotus species. Maderas Cienc Tecnol 20:571–578. https://doi.org/10.4067/S0718-221X2018005004501
Wilkinson A, Solan M, Taylor AFS et al (2010) Intraspecific diversity regulates fungal productivity and respiration. PLoS ONE 5(9):1–9. https://doi.org/10.1371/journal.pone.0012604
Acknowledgements
The study was supported by grants of the Slovak Research and Development Agency No. APVV-17-0644, APVV-16-0325 and grants of the Slovak Grant Agency KEGA No. 006TU Z-4/2019 and VEGA 1/0367/16.
Author information
Authors and Affiliations
Contributions
MP prepared substrates, inoculated fungi, wrote paper. PF Sr. run the experiments, interpreted the measurements. PF Jr. designed the experiment, run the statistical analysis, disccused the results. MP Jr. prepared fungal cultures and substrates, linguistic text editing. MŠ working with samples, laboratory measurements.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pavlík, M., Fleischer, P., Fleischer, P. et al. Evaluation of the Carbon Dioxide Production by Fungi Under Different Growing Conditions. Curr Microbiol 77, 2374–2384 (2020). https://doi.org/10.1007/s00284-020-02033-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02033-z