Abstract
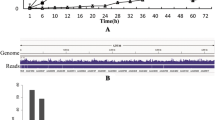
Expression of extracellular protease genes of Bacilli is subject to regulation by many positive and negative regulators. Here we analyzed 5′ regulatory regions of genes encoding proteolytic proteases AprBp, GseBp, and MprBp from Bacillus pumilus strain 3–19. Gfp fusion constructs with upstream genomic regions of different lengths were created for all three genes to identify their natural promoters (regulatory regions). Our results suggest that the aprBp gene, encoding the major subtilisin-like protease, has the most extensive promoter region of approximately 445 bp, while the minor protease genes encoding glutamyl endopeptidase (gseBp) and metalloproteinase (mprBp) are preceded by promoters of 150 and 250 bp in length, respectively. Promoter analysis of P aprBp -gfpmu3 and P gseBp -gfpmu3 reporter fusion constructs in degU and spo0A mutants indicates a positive regulatory effect of DegU and Spo0A on protease expression, while the disruption of abrB, sinR, and scoC repressor genes did not significantly affect promoter activities of all protease genes. On the other hand, the expression of P aprBp -gfpmu3 and P gseBp -gfpmu3 reporters increased 1.6- and 3.0-fold, respectively, in sigD-deficient cells, indicating that the prevention of motility gene expression promotes protease expression. Our results indicate that all examined regulators regulated serine proteases production in B. subtilis.



Similar content being viewed by others
References
Balaban NP, Mardanova AM, Sharipova MR, Gabdrakhmanova LA, Sokolova EA, Rudenskaya GN, Leshchinskaya IB (2004) Purification and characterization of serine proteinase 2 from Bacillus intermedius 3–19. BioChemistry 69:420–426. doi:10.1023/B:BIRY.0000026198.81752.f4
Birnboim HC, Doly J (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7:1513–1523
Bisicchia P, Botella E, Devine KM (2010) Suite of novel vectors for ectopic insertion of GFP, CFP and IYFP transcriptional fusions in single copy at the amyE and bglS loci in Bacillus subtilis. Plasmid 64:143–149. doi:10.1016/j.plasmid.2010.06.002
Botella E, Fogg M, Jules M, Piersma S, Doherty G, Hansen A, Denham EL, Le Chat L, Veiga P, Bailey K, Lewis PJ, van Dijl JM, Aymerich S, Wilkinson AJ, Devine KM (2010) pBaSysBioII: an integrative plasmid generating gfp transcriptional fusions for high-throughput analysis of gene expression in Bacillus subtilis. Microbiology 156:1600–1608. doi:10.1099/mic.0.035758-0
Chai Y, Kolter R, Losick R (2010) Reversal of an epigenetic switch governing cell chaining in Bacillus subtilis by protein instability. Mol Microbiol 78:218–229. doi:10.1111/j.1365-2958.2010.07335.x
Chai Y, Norman T, Kolter R, Losick R (2010) An epigenetic switch governing daughter cell separation in Bacillus subtilis. Genes Dev 24:754–765. doi:10.1101/gad.1915010
Chumsakul O, Takahashi H, Oshima T, Hishimoto T, Kanaya S, Ogasawara N, Ishikawa S (2011) Genome-wide binding profiles of the Bacillus subtilis transition state regulator AbrB and its homolog Abh reveals their interactive role in transcriptional regulation. Nucleic Acids Res 39:414–428. doi:10.1093/nar/gkq780
Cormack BP, Valdivia RH, Falkow S (1996) FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33–38
Danilova YV, Shagimardanova EI, Margulis AB, Toymentseva AA, Balaban NP, Rudakova NL, Rizvanov AA, Sharipova MR, Palotás A (2014) Bacterial enzymes effectively digest Alzheimer’s β-amyloid peptide. Brain Res Bull 108:113–117. doi:10.1016/j.brainresbull.2014.08.009
Ferrari E, Jarnagin AS, Schmidt BF (1993) Commercial production of extracellular enzymes. In: Sonenshein AL, Hoch JA, Losick R (eds) Bacillus subtilis and other Gram-positive bacteria. American Society for Microbiology, Washington, DC, pp 917–937
Fujita M, Losick R (2005) Evidence that entry into sporulation in Bacillus subtilis is governed by a gradual increase in the level and activity of the master regulator Spo0A. Genes Dev 19:2236–2244. doi:10.1101/gad.1335705
Gabdrakhmanova L, Vishniakov I, Sharipova M, Balaban N, Kostrov S, Leshchinskaya I (2005) Salt stress induction of glutamyl endopeptidase biosynthesis in Bacillus intermedius. Microbiol Res 160:233–242. doi:10.1016/j.micres.2004.05.005
Gupta R, Beg QK, Lorenz P (2002) Bacterial alkaline proteases: molecular approaches and industrial applications. Appl Microbiol Biotechnol 59:15–32. doi:10.1007/s00253-002-0975-y
Harwood CR, Cutting SM (1990) Molecular Biological Methods for Bacillus. Wiley, Chichester
Itskovich EL, Liutova LI, Balaban NP, Mardanova AM, Shakirov EV, Sharipova MR, Leshchinskaia IB, Rudenskaia GN (1998) Thrombolytic and anticoagulant properties of thiol-dependent proteinase from Bacillus intermedius 3–19. Vopr Med Khim 44:288–291
Jordan S, Rietkötter E, Strauch MA, Kalamorz F, Butcher BG, Helmann JD, Mascher T (2007) LiaRS-dependent gene expression is embedded in transition state regulation in Bacillus subtilis. Microbiology 153:2530–2540. doi:10.1099/mic.0.2007/006817-0
Kaiumov AR, Sabirova AR, Balaban NP, Mardanova AM, Il’inskaia ON, Kostrov SV, Sharipova MR (2008) Start codon in the serine proteinase gene from Bacillus intermedius. Mol Biol (Mosk) 42:117–122. doi:10.1007/s11008-008-1015-5.
Kallio PT, Fagelson JE, Hoch JA, Strauch MA (1991) The transition state regulator Hpr of Bacillus subtilis is a DNA-binding protein. J Biol Chem 266:13411–13417
Kayumov AR, Kirillova JM, Mikhailova EO, Balaban NP, Sharipova MR (2006) The prediction of regulation of subtilisin-like proteinase gene from Bacillus intermedius through its regulatory sequence analysis. BGRS-2006: Proceedings of the fifth international conference on Bioinformatics of Genome Regulation and Structure, Novosibirsk, July 16–22, pp 65–68.
Kearns DB, Losick R (2005) Cell population heterogeneity during growth of Bacillus subtilis. Genes Dev 19:3083–3094. doi:10.1101/gad.1373905
Kobayashi K (2007) Gradual activation of the response regulator DegU controls serial expression of genes for flagellum formation and biofilm formation in Bacillus subtilis. Mol Microbiol 66:395–409. doi:10.1111/j.1365-2958.2007.05923.x
Kodgire P, Dixit M, Rao KK (2006) ScoC and SinR negatively regulate epr by corepression in Bacillus subtilis. J Bacteriol 188:6425–6428. doi:10.1128/JB.00427-06
Kunst F, Rapoport G (1995) Salt stress is an environmental signal affecting degradative enzyme synthesis in Bacillus subtilis. J Bacteriol 177:2403–2407
Mäder U, Antelmann H, Buder T, Dahl MK, Hecker M, Homuth G (2002) Bacillus subtilis functional genomics: genome-wide analysis of the DegS-DegU regulon by transcriptomics and proteomics. Mol Genet Genom 268:455–467. doi:10.1007/s00438-002-0774-2
Mikhailova EO, Mardanova AM, Balaban NP, Rudenskaya GN, Ilyinskaya ON, Sharipova MR (2009) Biochemical properties of Bacillus intermedius subtilisin-like proteinase secreted by a Bacillus subtilis recombinant strain in its stationary phase of growth. BioChemistry 74(3):308–315. doi:10.1134/S0006297909030109
Mirouze N, Prepiak P, Dubnau D (2011) Fluctuations in spo0A transcription control rare developmental transitions in Bacillus subtilis. PLoS Genet 7(4):e1002048. doi:10.1371/journal.pgen.1002048
Msadek T (1999) When the going gets tough: survival strategies and environmental signaling networks in Bacillus subtilis. Trends Microbiol 7:201–207
Msadek T, Kunst F, Klier A, Rapoport G (1991) DegS-DegU and ComP-ComA modulator-effector pairs control expression of the Bacillus subtilis pleiotropic regulatory gene degQ. J Bacteriol 173:2366–2377
Ogura M, Matsuzawa A, Yoshikawa H, Tanaka T (2004) Bacillus subtilis SalA (YbaL) negatively regulates expression of scoC, which encodes the repressor for the alkaline exoprotease gene, aprE. J Bacteriol 186:3056–3064. doi:10.1128/JB.186.10.3056-3064.2004
Ogura M, Shimane K, Asai K, Ogasawara N, Tanaka T (2003) Binding of response regulator DegU to the aprE promoter is inhibited by RapG, which is counteracted by extracellular PhrG in Bacillus subtilis. Mol Microbiol 49:1685–1697. doi:10.1046/j.1365-2958.2003.03665.x
Ohsawa T, Tsukahara K, Ogura M (2009) Bacillus subtilis response regulator DegU is a direct activator of pgsB transcription involved in gamma-poly-glutamic acid synthesis. Biosci Biotechnol Biochem 73:2096–2102. doi:10.1271/bbb.9034
Sabirova AR, Rudakova NL, Balaban NP, Ilyinskaya ON, Demidyuk IV, Kostrov SV, Rudenskaya GN, Sharipova MR (2010) A novel secreted metzincin metalloproteinase from Bacillus intermedius. FEBS Lett 584:4419–4425. doi:10.1016/j.febslet.2010.09.049
Sánchez A, Olmos J (2004) Bacillus subtilis transcriptional regulators interaction. Biotechnol Lett 26:403–407
Shafikhani SH, Mandic-Mulec I, Strauch MA, Smith I, Leighton T (2002) Postexponential regulation of sin operon expression in Bacillus subtilis. J Bacteriol 184:564–571. doi:10.1128/JB.184.2.564-571.2002
Sharipova M, Balaban N, Kayumov A, Kirillova Y, Mardanova A, Gabdrakhmanova L, Leshchinskaya I, Rudenskaya G, Akimkina T, Safina D, Demidyuk I, Kostrov S (2008) The expression of the serine proteinase gene of Bacillus intermedius in Bacillus subtilis. Microbiol Res 163:39–50. doi:10.1016/j.micres.2006.03.003
Sharipova MR, Shagimardanova EI, Chastukhina IB, Shamsutdinov TR, Balaban NP, Mardanova AM, Rudenskaya GN, Demidyuk IV, Kostrov SV (2007) The expression of Bacillus intermedius glutamyl endopeptidase gene in Bacillus subtilis recombinant strains. Mol Biol Rep 34:79–87. doi:10.1007/s11033-006-9017-7
Sharipova MR, Toymentseva AA, Sabirova AR, Mukhametzianova AD, Akhmetova AI, Mardanova AM, Balaban NP (2011) New phylogenetic position Of Bacillus Intermedius 3–19 strain. Mikrobiologiia 80:424–426. doi:10.1134/S0026261711030192
Stepanov VG, Tirumalai MR, Montazari S, Checinska A, Venkateswaran K, Fox GE (2016) Bacillus pumilus SAFR-032 genome revisited: sequence update and re-annotation. PLoS One 11(6):e0157331. doi:10.1371/journal.pone.0157331
Strauch MA, Hoch JA (1993) Transition-state regulators: sentinels of Bacillus subtilis post-exponential gene expression. Mol Microbiol 7:337–342
Tsukahara K, Ogura M (2008) Characterization of DegU-dependent expression of bpr in Bacillus subtilis. FEMS Microbiol Lett 280:8–13. doi:10.1111/j.1574-6968.2007.01019.x
Zheng XL (2013) Structure-function and regulation of ADAMTS-13 protease. J Thromb Haemost 1:11–23. doi:10.1111/jth.12221
Acknowledgements
The authors express their gratitude to Dr. Yevgeniy V. Shakirov, University of Texas at Austin, USA, for reviewing the manuscript. The authors thank Sebastian Hübner for invaluable assistance with setting up the BioTek multimode microtiter plate reader and the corresponding data analysis. We thank Alexey Y. Gorbachev for his generous help with EMSA analysis. We are also grateful to the members of T. Mascher lab for B. subtilis mutant strains used in this study. The reported study was funded by RFBR, according to the research project No. 16-34-60198 mol_а_dk. Some genetic constructions were done within the DAAD-grant “Forschungsstipendien für Doktoranden und Nachwuchswissenschaftler” (325-A/09/84065). Work in the T. Mascher group was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG Grant MA2837/1-3). M.R. Sharipova was funded by Russian Science Foundation (RSF Grant No. 16-16-04062). The work is performed according to the Russian Government Program of Competitive Growth of Kazan Federal University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Toymentseva, A.A., Mascher, T. & Sharipova, M.R. Regulatory Characteristics of Bacillus pumilus Protease Promoters. Curr Microbiol 74, 550–559 (2017). https://doi.org/10.1007/s00284-017-1212-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-017-1212-3