Abstract
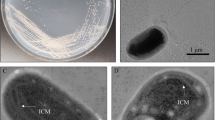
A fermentative, non-spore forming, motile, rod-shaped bacterium, designated strain MJ1T, was isolated from an RDX contaminated aquifer at a live-fire training site in Northwest NJ, United States. On the basis of 16S rRNA gene sequencing and DNA base composition, strain MJ1T was assigned to the Firmicutes. The DNA G+C content was 42.8 mol%. Fermentative growth was supported by glucose and citrate in a defined basal medium. The bacterium is a strict anaerobe that grows between at pH 6.0 and pH 8.0 and 18 and 37 °C. The culture did not grow with hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) as the electron acceptor or mineralize RDX under these conditions. However, MJ1T transformed RDX into MNX, methylenedinitramine, formaldehyde, formate, ammonium, nitrous oxide, and nitrate. The nearest phylogenetic relative with a validly published name was Desulfotomaculum guttoideum (95 % similarity). However, MJ1T was also related to Clostridium celerecrescens DSM 5628 (95 %), Clostridium indolis DSM 755 (94 %), and Clostridium sphenoides DSM 632 (94 %). DNA:DNA hybridization with these strains was between 6.7 and 58.7 percent. The dominant cellular fatty acids (greater than 5 % of the total, which was 99.0 % recovery) were 16:0 fatty acid methyl ester (FAME) (32.12 %), 18:1cis 11 dimethyl acetal (DMA) (16.47 %), 16:1cis 9 DMA (10.28 %), 16:1cis 9 FAME (8.10 %), and 18:1cis 9 DMA (5.36 %). On the basis of morphological, physiological, and phylogenetic data, Clostridium geopurificans is proposed as a new species in genus Clostridium, with strain MJ1T as the type strain.


Similar content being viewed by others
References
Adrian NR, Arnett CM (2006) Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) serves as a carbon and energy source for a mixed culture under anaerobic conditions. Curr Microbiol 53:129–134
Adrian NR, Arnett CM, Hickey RF (2003) Stimulating the anaerobic biodegradation of explosives by the addition of hydrogen or electron donors that produce hydrogen. Water Res 37:3499–3507
Bhushan B, Halasz A, Hawari J (2006) Effect of iron(III), humic acids and anthraquinone-2,6-disulfonate on biodegradation of cyclic nitramines by Clostridium sp EDB2. J Appl Microbiol 100:555–563
Bhushan B, Halasz A, Thiboutot S, Ampleman G, Hawari J (2004) Chemotaxis-mediated biodegradation of cyclic nitramine explosives RDX, HMX, and CL-20 by Clostridium sp. EDB2. Biochem Biophys Res Comm 316:816–821
Castro HF, Williams NH, Ogram A (2000) Phylogeny of sulfate-reducing bacteria. FEMS Microbiol Ecol 31:1–9
Collins MD, Lawson PA, Willems A, Cordoba JJ, Fernandezgarayzabal J, Garcia P, Cai J, Hippe H, Farrow JAE (1994) The phylogeny of the genus Clostridium—proposal of five new genera and 11 new species combinations. Int J Syst Bacteriol 44:812–826
Cullum HE, McGavigan C, Uttley CZ, Stroud MAM, Warren DC (2004) A second survey of high explosives traces in public places. J Forensic Sci 49:684–690
DOE (1998) “Treatment of HMX and RDX Contamination, Amarillo National Resource Center for Plutonium, March 1998, ANRCP-1998-2,”
Finneran KT, Johnsen CV, Lovley DR (2003) Rhodoferax ferrireducens sp. nov., a Psychrotolerant, facultatively anaerobic bacterium that oxidizes acetate with the reduction of Fe(III). Int J Syst Evol Microbiol 53:669–673
Fournier D, Halasz A, Spain J, Firuasek P, Hawari J (2002) Determination of key metabolites during biodegradation of hexahydro-1,3,5-trinitro-triazine with Rhodococcus sp. Strain DN22. Appl Environ Microbiol 68:166–172
Haas R, Schreiber I, Low EU, Stork G (1990) Conception for the investigation of contaminated munition plants. Fresenis’ J Analy Chem 338:41–45
Hayashi H, Sakamoto M, Benno Y (2004) Evaluation of three different forward primers by terminal restriction fragment length polymorphism analysis for determination of fecal Bifidobacterium spp. in healthy subjects. Microbiol Immunol 48:1–6
Kampfer P, Kroppenstedt RM (1996) Numerical analysis of fatty acid patterns of coryneform bacteria and related taxa. Can J Microbiol 42:989–1005
Kuykendall LD, Roy MA, Oneill JJ, Devine TE (1988) Fatty-acids, antibiotic-resistance, and deoxyribonucleic-acid homology groups of Bradyrhizobium japonicum. Int J Syst Bacteriol 38:358–361
Kwon MJ, Finneran KT (2008) Distribution of products and mineralization potential for hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) in abiotic versus biological degradation pathways with anthraquinone-2,6-disulfonate (AQDS) and Geobacter metallireducens. Biodegradation 19:705–715
Kwon MJ, Finneran KT (2008) Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and Octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) biodegradation kinetics amongst several Fe(III)-reducing genera. Soil Sediment Contam 17:189–203
Lanyi B (1987) Classical and rapid identification methods for medically important bacteria. In: Colwell RR, Grigorova R (eds) Methods in microbiology, vol 19. Academic Press, New York, pp 1–67
Lawson PA, Llopperez P, Hutson RA, Hippe H, Collins MD (1993) Towards a phylogeny of the Clostridia based on 16S ribosomal-RNA sequences. FEMS Microbiol Lett 113:87–92
Lovley DR, Giovannoni SJ, White DC, Champine JE, Philips EJP, Gorby YA, Goodwin S (1993) Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch Microbiol 159:336–344
Lovley DR, Phillips EJP (1988) Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol 54:1472–1480
Miller LT (1982) Single derivatization method for routine analysis of bacterial whole-cell fatty-acid methyl-esters, including hydroxy-acids. J Clin Microbiol 16:584–586
Palop ML, Valles S, Pinaga F, Flors A (1991) Characterization of cellulase and xylanase activities of Clostridium celerecrescens. J Chem Technol Biotechnol 51:105–114
Palop ML, Valles S, Pinaga F, Flors A (1989) Isolation and characterization of an anaerobic, cellulolytic bacterium, Clostridium celerecrescens sp-nov. Int J Syst Bacteriol 39:68–71
Park DH, Laivenieks M, Guettler MV, Jain MK, Zeikus JG (1999) Microbial utilization of electrically reduced neutral red as the sole electron donor for growth and metabolite production. Appl Environ Microbiol 65:2912–2917
Park DH, Zeikus JG (2000) Electricity generation in microbial fuel cells using neutral red as an electronophore. Appl Environ Microbiol 66:1292–1297
Park DH, Zeikus JG (1999) Utilization of electrically reduced neutral red by Actinobacillus succinogenes: physiological function of neutral red in membrane-driven fumarate reduction and energy conservation. J Bacteriol 181:2403–2410
Pennington JC, Brannon JM (2002) Environmental fate of explosives. Thermochim Acta 384:163–172
Rainey FA, Hollen BJ, Small A (2009) Bergey’s manual of systmatic bacteriology, genus I, Clostridium, vol 3, 2nd edn. Springer, New York
Seth-Smith HMB, Rosser SJ, Basran A, Travis ER, Dabbs ER, Nicklin S, Bruce NC (2002) Cloning, sequencing, and characterization of the hexahydro-1,3,5-trinitro-1,3,5-triazine degradation gene cluster from Rhodococcus rhodochrous. Appl Environ Microbiol 68:4764–4771
Sierra G (1957) A simple method for the detection of lipolytic activity of microorganisms and some observations on the influence of the contact between cells and fatty substrates. Antonie Van Leeuwenhoek 23:15–22
Spalding RF, Fulton JW (1988) Groundwater munition residues and nitrate near Grand Island, Nebraska. USA J Contam Hydrol 2:139–153
Stackenbrandt E, Sproer C, Rainey FA, Burghardt J, Pauker O, Hippe H (1997) Phylogenetic analysis of the genus Desulfotomaculum: evidence for the misclassification of Desulfotomaculum guttoideum and description of Desulfotomaculum orientis as Desulfosporosinus orientis gen. nov., comb. nov. Int J Syst Bacteriol 47:1134–1139
Thompson KT, Crocker FH, Fredrickson HL (2005) Mineralization of the cyclic nitramine explosive hexahydro-1,3,5-trinitro-1,3,5-triazine by Gordonia and Williamsia spp. Appl Environ Microbiol 71:8265–8272
Walther R, Hippe H, Gottschalk G (1977) CItrate, a specific substrate for isolation of Clostridium sphenoides. Appl Environ Microbiol 33:955–962
Zhao J-S, Greer CW, Thiboutot S, Ampleman G, Hawari J (2004) Biodegradation of the nitramine explosives hexahydro-1,3,5-trinitro-1,3,5-triazine and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine in cold marine sediment under anaerobic and oligotrophic conditions. Can J Microbiol/Rev Can Microbiol 50:91–96
Zhao J-S, Halasz A, Paquet L, Beaulieu C, Hawari J (2002) Biodegradation of hexahydro-1,3,5-trinitro-1,3,5-triazine and its mononitroso derivative hexahydro-1-nitroso-3,5-dinitro-1,3,5-triazine by Klebsiella pneumoniae Strain SCZ-1 isolated from an anaerobic sludge. Appl Environ Microbiol 68:5336–5341
Zhao JS, Paquet L, Halasz A, Hawari J (2003) Metabolism of hexahydro-1,3,5-trinitro-1,3,5-triazine through initial reduction to hexahydro-1-nitroso-3,5-dinitro-1,3,5-triazine followed by denitration in Clostridium bifermentans HAW-1. Appl Microbiol Biotechnol 63:187–193
Zhao JS, Spain J, Thiboutot S, Ampleman G, Greer C, Hawari J (2004) Phylogeny of cyclic nitramine-degrading psychrophilic bacteria in marine sediment and their potential role in the natural attenuation of explosives. FEMS Microbiol Ecol 49:349–357
Zhou JZ, He Q, Hemme CL, Mukhopadhyay A, Hillesland K, Zhou AF, He ZL, Van Nostrand JD, Hazen TC, Stahl DA, Wall JD, Arkin AP (2011) How sulphate-reducing microorganisms cope with stress: lessons from systems biology. Nat Rev Microbiol 9:452–466
Acknowledgments
We thank Manish Kumar (Illinois) for help with SEM photos. We also thank Paul Hatzinger of Shaw Environmental and Scott Drew of GeoSyntec Consultants for acquiring the field samples. M.J. Kwon was supported by both DoD SERDP (Project ER-1377) and Korea Ministry of Environment as “The GAIA Project-2013000540005”, while working towards the completion of this project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kwon, M.J., Wei, N., Millerick, K. et al. Clostridium geopurificans Strain MJ1 sp. nov., A Strictly Anaerobic Bacterium that Grows via Fermentation and Reduces the Cyclic Nitramine Explosive Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX). Curr Microbiol 68, 743–750 (2014). https://doi.org/10.1007/s00284-014-0531-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-014-0531-x