Abstract
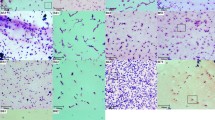
To explain the association of Bacillus thuringiensis (Bt) with animal feces, an ecological analysis in chickens was conducted by introducing a cry − strain marked by production of green fluorescent protein (GFP). After feeding with the tagged Bt strains, the feces of the tested chickens were collected at different times, isolated, and the morphology of Bt was observed. It was shown that Bt strain HD-73GFP in spore form could be isolated from feces of chickens for a period of 13 d, and then it disappeared thereafter. Bt could be detected only up to day 4 (but not thereafter), when chickens were fed with vegetative cells of HD-73GFP. To confirm the source of newly isolated strains, the gfp gene was examined by polymerase chain reaction (PCR), which showed that all the isolated strains harbored the marker gene. Recent data from isolation and PCR had suggested that fecal Bt strains had originated from food. Chicken tissues were thus dissected to isolate Bt strains and to investigate whether Bt could be located in vivo. Bt was located within the duodenum in spore form. Compared to the morphology of the isolated strains at different growth times, the growth rates of all the tested Bt had little changes when passing through the digestive system to the feces. Dissection of the chickens confirmed that Bt was safe for the tested animal.



Similar content being viewed by others
References
Ammons DR, Reyna A, Granados JC, Samlal MS, Rampersad JN (2009) An investigation of Bacillus thuringiensis in rectal-collected fecal samples of cows. Curr Microbiol 59:532–536
Bernstein IL, Bernstein JA, Miller M, Bernstein DI, Lummus Z, Selgrade MK, Doerfler DL, Seligy VL (1999) Immune responses in farm workers after exposure to Bacillus thuringiensis pesticides. Environ Health Perspect 107(7):575–582
Bishop AH, Johnson C, Perani M (1999) The safety of Bacillus thuringiensis to mammals investigated by oral and subcutaneous dosage. World J Microbiol Biotechnol 15:375–380
Bizzarri MF, Bishop AH (2007) Recovery of Bacillus thuringiensis in vegetative form from the phylloplane of clover (Trifolium hybridum) during a growing season. J Invertebr Pathol 94:38–47
Bizzarri MF, Bishop AH (2008) The ecology of Bacillus thuringiensis on the phylloplane: colonization from soil, plasmid transfer, and interaction with larvae of Pieris brassicae. Microb Ecol 56(1):133–139
Hansen BM, Salamitou S (2000) Virulence of Bacillus thuringiensis. In: Charles J-F, Delecluse A, Nielsen-LeRoux C (eds) Entomopathogenic bacteria: from laboratory to field application. Kluwer, Dordrecht, pp 41–64
Hendriksen NB, Hansen BM (2002) Long-term survival and germination of Bacillus thuringiensis var. kurstaki in a field trial. Can J Microbiol 48(3):256–261
Hernández-Rodríguez CS, Ferré J (2009) Ecological distribution and characterization of four collections of Bacillus thuringiensis strains. J Basic Microbiol 49(2):152–157
Ichimatsu T, Mizuki E, Nishimura K, Akao T, Saitoh H, Higuchi K (2000) Occurrence of Bacillus thuringiensis in fresh waters of Japan. Curr Microbiol 40:217–220
Iriarte J, Porcar M, Lecadet M, Caballero P (2000) Isolation and characterization of Bacillus thuringiensis strains from aquatic environments in Spain. Curr Microbiol 40:402–408
Ishiwata S (1901) On a kind of severe flacherie (sotto disease). Dainihon Sanshi Kaiho 114:1–5
Jensen GB, Hansen BM, Eilenbery J, Mahillon J (2003) The hidden lifestyles of Bacillus cereus and relatives. Environ Microbiol 5:631–640
Jensen GB, Larsen P, Jacobsen BL, Madsen B, Smidt L, Andrup L (2002) Bacillus thuringiensis in fecal samples from greenhouse workers after exposure to B. thuringiensis-based pesticides. Appl Environ Microbiol 68(10):4900–4905
Jensen GB, Larsen P, Jacobsen BL, Madsen B, Wilcks A, Smidt L, Andrup L (2002) Isolation and characterization of Bacillus cereus-like bacteria from faecal samples from greenhouse workers who are using Bacillus thuringiensis-based insecticides. Int Arch Occup Environ Health 75(3):191–196
Lee DH, Cha IH, Woo DS, Ohba M (2003) Microbial ecology of Bacillus thuringiensis: fecal populations recovered from wildlife in Korea. Can J Microbiol 49(7):465–471
Lee DH, Machii J, Ohba M (2002) High frequency of Bacillus thuringiensis in feces of herbivorous animals maintained in a zoological garden in Japan. Appl Entomol Zool 37(4):509–516
Lee DH, Shisa N, Wasano N, Ohgushi A, Ohba M (2003) Characterization of flagellar antigens and insecticidal activities of Bacillus thuringiensis populations in animal feces. Curr Microbiol 46(4):287–290
Lereclus D, Arantès O, Chaufaux J, Lecadet M (1989) Transformation and expression of a cloned delta-endotoxin gene in Bacillus thuringiensis. FEMS Microbiol Lett 51(1):211–217
Maheswaran S, Sreeramanan S, Josephine CMR, Marimuthu K, Xavier R (2010) Occurrence of Bacillus thuringiensis in faeces of herbivorous farm animals. Afr J Biotechnol 47(9):8013–8019
Meadows MP, Ellis DJ, Butt J, Jarrett P, Burges HD (1992) Distribution, frequency and diversity of Bacillus thuringiensis in an animal feed mill. Appl Environ Microbiol 58(4):1344–1350
Monnerat RG, Soares CM, Capdeville G, Jones G, Martins ES, Praça L, Cordeiro BA, Braz SV, dos Santos RC, Berry C (2009) Translocation and insecticidal activity of Bacillus thuringiensis living inside of plants. Microb Biotechnol 2(4):512–520
Ohba M, Lee DH (2003) Bacillus thuringiensis associated with faeces of the Kerama-jika, Cervus nippon keramae, a wild deer indigenous to the Ryukyus, Japan. J Basic Microbiol 43(2):158–162
Pedersen JC, Damgaard PH, Eilenberg E, Hansen BM (1995) Dispersal of Bacillus thuringiensis var. kurstaki in an experimental cabbage field. Can J Microbiol 41(2):118–125
Raymond B, Elliot SL, Ellis RJ (2008) Quantifying the reproduction of Bacillus thuringiensis HD-1 in cadavers and live larvae of Plutella xylostella. J Invertebr Pathol 98(3):307–313
Smith RA, Gouche GA (1991) The phylloplane as a source of Bacillus thuringiensis variants. Appl Environ Microbiol 57(1):311–315
Swiecicka I, Fiedoruk K, Bednarz G (2002) The occurrence and properties of Bacillus thuringiensis isolated from free-living animals. Lett Appl Microbiol 34(3):194–198
Wu CB, Guan Y, Qiu JJ, Huang YM, Zhang AH (2009) Biological characteristics of Bacillus thuringiensis isolated from the feces of a Sika deer and identification of its cry-type genes. J Econ Anim 13(2):72–76 (Chinese with English abstract)
Zhang L, Huang E, Lin J, Gelbič I, Zhang Q, Guan Y, Huang T, Guan X (2010) A novel mosquitocidal Bacillus thuringiensis strain LLP29 isolated from the phylloplane of Magnolia denudata. Microbiol Res 165:133–141
Zhang B, Jiang DD, Zhou WW, Hao HK, Niu TG (2009) Isolation and characterization of a new Bacillus sp. 50-3 with highly alkaline keratinase activity from Calotes versicolor faeces. World J Microbiol Biotechnol 25(4):583–590
Acknowledgments
This study has been supported by the National Natural Science Foundation of China (Grant No. 31071745), the Science Foundation of the Ministry of Education of China (Grant Nos. 20093515110010 and 20093515120010), the Transformation Fund for Agricultural Science and Technology Achievements (Grant No. 2010GB2C400212), Education Department Foundation of Fujian Province Distinguished Young Scholars in Universities of Fujian Province (Grand No. JA12092), Grant No. 2B08003 from the Ministry of Education of the Czech Republic, and Project No. Z50070508 of the Institute of Entomology of the Academy of Sciences of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yan Peng: Co-first author.
Rights and permissions
About this article
Cite this article
Zhang, L., Peng, Y., Wu, S. et al. Microbial Ecology and Association of Bacillus thuringiensis in Chicken Feces Originating from Feed. Curr Microbiol 65, 784–791 (2012). https://doi.org/10.1007/s00284-012-0231-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-012-0231-3