Abstract
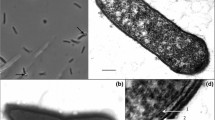
A thermophilic anaerobic bacterium (strain TH7C1T) was isolated from the hydrothermal hot spring of Guelma in the northeast of Algeria. Strain TH7C1T stained Gram-positive, was a non-motile rod appearing singly, in pairs, or as long chains (0.7–1 × 2–6 μm2). Spores were never observed. It grew at temperatures between 55 and 75°C (optimum 65°C) and at pH between 6.2 and 8.3 (optimum 6.9). It did not require NaCl for growth, but tolerated it up to 5 g l−1. Strain TH7C1T is an obligatory heterotroph fermenting sugars including glucose, galactose, lactose, raffinose, fructose, ribose, xylose, arabinose, maltose, mannitol, cellobiose, mannose, melibiose, saccharose, but also xylan, and pyruvate. Fermentation of sugars only occurred in the presence of yeast extract (0.1%). The end-products from glucose fermentation were acetate, lactate, ethanol, CO2, and H2. Nitrate, nitrite, thiosulfate, elemental sulfur, sulfate, and sulfite were not used as electron acceptors. The G+C content of the genomic DNA was 44.7 mol% (HPLC techniques). Phylogenetic analysis of the small-subunit ribosomal RNA (rRNA) gene sequence indicated that strain TH7C1T was affiliated to Firmicutes, order Clostridiales, family Caldicoprobacteraceae, with Caldicoprobacter oshimai (98.5%) being its closest relative. Based on phenotypic, phylogenetic, and genetic characteristics, strain TH7C1T is proposed as a novel species of genus Caldicoprobacter, Caldicoprobacter algeriensis, sp. nov. (strain TH7C1T = DSM 22661T = JCM 16184T).


Similar content being viewed by others
References
Altschul SF, Madden TL, Schaffer AA et al (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Asao M, Madigan MT (2009) Family IV. Heliobacteriaceae. In: De Vos P, Garrity GM, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer KH, Whitman B (eds) The Firmicutes, vol 3, 2nd edn. Springer, Dordrecht, pp 913–916
Balch WE, Fox GE, Magrum LJ, Woese CR et al (1979) Methanogens: reevaluation of a unique biological group. Microbiol Rev 43:260–296
Ben Dhia Thabet O, Fardeau ML, Joulian C et al (2004) Clostridium tunisiense sp. nov., a new proteolytic, sulphur-reducing bacterium isolated from an olive mill wastewater contaminated by phosphogypse. Anaerobe 10:185–190
Benson DA, Karsch-Mizrachi I, Lipman DJ et al. (2008) GenBank. Nucleic Acids Res 36(Database issue): D25–D30. doi:10.1093/nar/gkn979
Cashion P, Holder-Franklin MA, McCully J et al (1977) A rapid method for the base ratio determination of bacterial DNA. Anal Biochem 81:461–466
Cole JR, Wang Q, Cardenas E, Fish J et al. (2009) The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 37(Database issue): D141–D145. doi:10.1093/nar/gkn879
Collins MD, Lawson PA, Willems A et al (1994) The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int J Syst Bacteriol 44:812–826
Engle M, Li Y, Rainey F et al (1996) Thermobrachium celere gen. nov., sp. nov., a rapidly growing thermophilic, alkalitolerant, and proteolytic obligate anaerobe. Int J Syst Bacteriol 46:1025–1033
Esaki T (2009) Family VI. Peptococcaceae. In: De Vos P, Garrity GM, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer KH, Whitman B (eds) The Firmicutes, vol 3, 2nd edn. Springer, Dordrecht, pp 969–971
Escara JF, Hutton JR (1980) Thermal stability and renaturation of DNA in dimethylsulphoxide solutions: acceleration of renaturation rate. Biopolymers 19:1315–1327
Fardeau ML, Ollivier B, Cayol JL (2009) Genus III. Caldanaerobacter. In: De Vos P, Garrity GM, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer KH, Whitman B (eds) The Firmicutes, vol 3, 2nd edn. Springer, Dordrecht, pp 1241–1244
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hobel CFV, Marteinsson VT, Hauksdóttir S et al (2004) Use of low nutrient enrichments to access novel amylase genes in silent diversity of thermophiles. World J Microbiol Biotechnol 20:801–809
Hungate RE (1969) A roll tube method for the cultivation of strict anaerobes. In: Norris JR, Ribbons DW (eds) Methods in microbiology, vol 3B. Academic Press, New York, pp 117–132
Jukes TH, Cantor CR (1969) Evolution of protein molecules. In: Munro HN (ed) Mammalian protein metabolism. Academic Press, New York, pp 21–132
Kecha M, Benallaoua S, Touzel JP et al (2007) Biochemical and phylogenetic characterization of a novel terrestrial hyperthermophilic archaeon pertaining to the genus Pyrococcus from an Algerian hydrothermal hot spring. Extremophiles 11:65–73
Mesbah M, Premachandran U, Whitman WB (1989) Precise measurement of the G+C content of deoxyribonucleic acid by high-performance liquid chromatography. Int J Syst Bacteriol 39:159–167
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem 31:426–442
Onyenwoke RU, Wiegel J (2009) Genus II. Thermoanaerobacterium. In: De Vos P, Garrity GM, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer KH, Whitman B (eds) The Firmicutes, vol 3, 2nd edn. Springer, Dordrecht, pp 1279–1287
Onyenwoke RU, Wiegel J (2009) Genus I. Thermoanaerobacter. In: De Vos P, Garrity GM, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer KH, Whitman B (eds) The Firmicutes, vol 3, 2nd edn. Springer, Dordrecht, pp 1225–1239
Patel BKC, Monk C, Littleworth H et al (1987) Clostridium fervidus sp. nov., a new chemoorganotrophic acetogenic thermophile. Int J Syst Bacteriol 37:123–126
Rayney FA (2009) Order I. Clostridiales. In: De Vos P, Garrity GM, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer KH, Whitman B (eds) The Firmicutes, vol 3, 2nd edn. Springer, Dordrecht, p 736
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:405–425
Sekiguchi Y (2009) Family Syntrophomonadaceae. In: De Vos P, Garrity GM, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer KH, Whitman B (eds) The Firmicutes, vol 3, 2nd edn. Springer, Dordrecht, pp 1044–1045
Slobodkin AI, Tourova TP, Kostrikina NA et al (2006) Tepidimicrobium ferriphilum gen. nov., sp. nov., a novel moderately thermophilic, Fe(III)-reducing bacterium of the order Clostridiales. Int J Syst Evol Microbiol 56:369–372
Wagner ID, Wiegel J (2008) Diversity of thermophilic anaerobes. Ann N Y Acad Sci 1125:1–43
Wayne LG, Brenner DJ, Colwell RR et al (1987) Report of the Ad Hoc committee on reconciliation of approaches to bacterial systematics. Int J Syst Bacteriol 37:463–464
Weisburg WG, Barns SM, Pelletier DA et al (1991) 16S Ribosomal DNA Amplification for phylogenetic study. J Bacteriol 173:697–703
Wiegel J (2009) Order III. Thermoanaerobacterales. In: De Vos P, Garrity GM, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer KH, Whitman B (eds) The Firmicutes, vol 3, 2nd edn. Springer, Dordrecht, p 1224
Wiegel J (2009) Family I. Clostridiaceae. In: De Vos P, Garrity GM, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer KH, Whitman B (eds) The Firmicutes, vol 3, 2nd edn. Springer, Dordrecht, pp 736–738
Winker S, Woese CR (1991) A definition of the domains, Archaea, Bacteria and Eucarya in terms of small subunit ribosomal RNA characteristics. Syst Appl Microbiol 13:161–165
Yokoyama H, Wagner ID, Wiegel J (2010) Caldicoprobacter oshimai gen. nov., sp. nov., an anaerobic, xylanolytic, extremely thermophilic bacterium isolated from sheep faeces, and proposal of Caldicoprobacteraceae fam. nov. Int J Syst Evol Microbiol 60:67–71
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bouanane-Darenfed, A., Fardeau, ML., Grégoire, P. et al. Caldicoprobacteralgeriensis sp. nov. a New Thermophilic Anaerobic, Xylanolytic Bacterium Isolated from an Algerian Hot Spring. Curr Microbiol 62, 826–832 (2011). https://doi.org/10.1007/s00284-010-9789-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-010-9789-9