Abstract
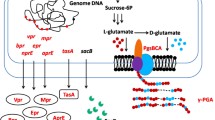
Bacillusamyloliquefaciens C06, a potential agent in biological preservation of post-harvest fruit, was found to secrete extra-cellular γ-polyglutamic acid (γ-PGA) in liquid culture. In this work, M306, a transposon mutant of B. amyloliquefaciens C06, defective in forming structured colony and displaying enhanced ability of producing γ-PGA, was obtained. Inverse PCR and quantitative reverse transcription PCR (qRT-PCR) analysis demonstrated that the defective phenotype in M306 was associated with an ORF showing high similarity to RBAM_034550 from B. amyloliquefaciens FZB42. In this paper, the ORF was designated pbrA, standing for γ-PGA production and biofilm formation regulatory factor. qRT-PCR analysis also indicated that pbrA down-regulated mRNA expression of epsD and yqxM, the crucial genes involved in biofilm formation, but affected little on expression of ywtB, the gene directing γ-PGA synthesis. Evaluations in γ-PGA productivity of wild-type C06 and its mutants C06ΔepsA and C06ΔtasA, respectively, deficient in producing exopolysaccharides (EPS) and TasA, revealed that γ-PGA overproduction in M306 was probably due to the redistributed metabolic flux caused by defective production of EPS.






Similar content being viewed by others
References
Ashiuchi M, Soda K, Misono H (1999) A poly-γ-glutamate synthetic system of Bacillus subtilis IFO 3336: gene cloning and biochemical analysis of poly-γ-glutamate produced by Escherichia coli clone cells. Biochem Biophys Res Commun 263:6–12
Bertani G (1951) Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol 62:293–300
Birrer GA, Cromwick AM, Gross RA (1994) γ-poly (glutamic acid) formation by Bacillus licheniformis 9945a: physiological and biochemical studies. Int J Biol Macromol 16:265–275
Bovarnick M (1942) The formation of extracellular d (-)-glutamic acid polypeptide by Bacillus subtilis. J Biol Chem 145:415–424
Branda SS, Gonzalez-Pastor JE, Ben-Yehuda S, Losick R, Kolter R (2001) Fruiting body formation by Bacillus subtilis. Proc Natl Acad Sci USA 98:11621–11626
Branda SS, Gonzalez-Pastor JE, Dervyn E, Ehrlich SD, Losick R, Kolter R (2004) Genes involved in formation of structured multicellular communities by Bacillus subtilis. J Bacteriol 186:3970–3979
Branda SS, Vik S, Friedman L, Kolter R (2005) Biofilms: the matrix revisited. Trends Microbiol 13:20–26
Chen XH, Koumoutsi A, Scholz R, Eisenreich A, Schneider K, Heinemeyer I, Morgenstern B, Voss B, Hess WR, Reva O (2007) Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nat Biotechnol 25:1007–1014
Cromwick AM, Birrer GA, Gross RA (1996) Effects of pH and aeration on γ-poly (glutamic acid) formation by Bacillus licheniformis in controlled batch fermentor cultures. Biotechnol Bioeng 50:222–227
Cromwick AM, Gross RA (1995) Effects of manganese (II) on Bacillus licheniformis ATCC 9945A physiology and γ-poly (glutamic acid) formation. Int J Biol Macromol 17:259–267
Goto A, Kunioka M (1992) Biosynthesis and hydrolysis of poly (γ-glutamic acid) from Bacillus subtilis IFO 3335. Biosci Biotechnol Biochem 56:1031–1035
Hasebe K, Inagaki M (1999) Preparation composition for external use containing poly (γ-glutamic acid) and vegetable extract in combination. Japanese Patent No. 11,240,827
Ivanovics G, Bruckner V (1937) Chemische und immunologische Studien uber den Mechanismus der Milzbrandinfektion und Immunitat die chemische Struktur der Kapel-substanz des Milzbrand-bacillus und der serolisch identischen spezifichen Substanz des Bacillus mesentericus. Z Immun Exp Ther 90:304–310
Kearns DB, Chu F, Branda SS, Kolter R, Losick R (2005) A master regulator for biofilm formation by Bacillus subtilis. Mol Microbiol 55:739–749
Konno A, Taguchi T, Yamaguchi T (1989) Bakery products and noodles containing polyglutamic acid. US Patent No. 4,888,193
Kubota H, Matsunobu T, Uotani K, Takebe H, Satoh A, Tanaka T, Taniguchi M (1993) Production of poly (γ-glutamic acid) by Bacillus subtilis F-2-01. Biosci Biotechnol Biochem 44:501–506
Kurosaki T, Kitahara T, Fumoto S, Nishida K, Nakamura J, Niidome T, Kodama Y, Nakagawa H, To H, Sasaki H (2009) Ternary complexes of pDNA, polyethylenimine, and gamma-polyglutamic acid for gene delivery systems. Biomaterials 30:2846–2853
Le Breton Y, Mohapatra NP, Haldenwang WG (2006) In vivo random mutagenesis of Bacillus subtilis by use of TnYLB-1, a mariner-based transposon. Appl Environ Microbiol 72:327–333
Lemon KP, Earl AM, Vlamakis HC, Aguilar C, Kolter R (2008) Biofilm development with an emphasis on Bacillus subtilis. Curr Top Microbiol Immunol 322:1–16
Leonard CG, Housewright RD, Thorne CB (1958) Effects of some metallic ions on glutamyl polypeptide synthesis by Bacillus subtilis. J Bacteriol 76:499–503
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Manocha B, Margaritis A (2008) Production and characterization of γ-polyglutamic acid nanoparticles for controlled anticancer drug release. Crit Rev Biotechnol 28:83–99
Marchler-Bauer A, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, Geer LY, Geer RC, Gonzales NR, Gwadz M et al (2009) CDD: specific functional annotation with the Conserved Domain Database. Nucleic Acids Res 37:205–210
Ohsawa T, Tsukahara K, Ogura M (2009) Bacillus subtilis response regulator DegU is a direct activator of pgsB transcription involved in gamma-poly-glutamic acid synthesis. Biosci Biotechnol Biochem 73:2096–2102
Osera C, Amati G, Calvio C, Galizzi A (2009) SwrAA activates poly-γ-glutamate synthesis in addition to swarming in Bacillus subtilis. Microbiology 155:2282–2287
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Shih IL, Van YT (2001) The production of poly-(γ-glutamic acid) from microorganisms and its various applications. Bioresour Technol 79:207–225
Spizizen J (1958) Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc Natl Acad Sci USA 44:1072–1078
Stanley NR, Lazazzera BA (2005) Defining the genetic differences between wild and domestic strains of Bacillus subtilis that affect poly-gamma-dl-glutamic acid production and biofilm formation. Mol Microbiol 57:1143–1158
Su Y, Li X, Liu Q, Hou Z, Zhu X, Guo X, Ling P (2010) Improved poly-γ-glutamic acid production by chromosomal integration of the Vitreoscilla hemoglobin gene (vgb) in Bacillus subtilis. Bioresour Technol 101:4733–4736
Taniguchi M, Kato K, Shimauchi A, Ping X, Nakayama H, Fujita K-I, Tanaka T, Tarui Y, Hirasawa E (2005) Proposals for wastewater treatment by applying flocculating activity of cross-linked poly-γ-glutamic acid. J Biosci Bioeng 99:245–251
Troy FA (1973) Chemistry and biosynthesis of the poly(-D-glutamyl) capsule in Bacillus licheniformis. I. Properties of the membrane-mediated biosynthetic reaction. J Biol Chem 248:305–315
Wu Q, Xu H, Shi N, Yao J, Li S, Ouyang P (2008) Improvement of poly(γ-glutamic acid) biosynthesis and redistribution of metabolic flux with the presence of different additives in Bacillus subtilis CGMCC 0833. Appl Microbiol Biotechnol 79:527–535
Wu Q, Xu H, Xu L, Ouyang P (2006) Biosynthesis of poly (γ-glutamic acid) in Bacillus subtilis NX-2: regulation of stereochemical composition of poly (γ-glutamic acid). Process Biochem 41:1650–1655
Xu H, Jiang M, Li H, Lu D, Ouyang P (2005) Efficient production of poly (γ-glutamic acid) by newly isolated Bacillus subtilis NX-2. Process Biochem 40:519–523
Zhou T, Schneider KE, Li XZ (2008) Development of biocontrol agents from food microbial isolates for controlling post-harvest peach brown rot caused by Monilinia fructicola. Int J Food Microbiol 126:180–185
Acknowledgements
This research is funded by Agriculture and Agri-food Canada and also supported by grants from National Natural Science Fund of China (30570041), the National 863 Program of China (2006AA10Z172), the Program of International Science and Technology Cooperation (2009DFA32740), and the Special Nonprofit Scientific Research Program, P. R. China (3–23). J. Liu received a graduate scholarship from the China Scholarship Council.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, J., Ma, X., Wang, Y. et al. Depressed Biofilm Production in Bacillus amyloliquefaciens C06 Causes γ-Polyglutamic Acid (γ-PGA) Overproduction. Curr Microbiol 62, 235–241 (2011). https://doi.org/10.1007/s00284-010-9696-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-010-9696-0