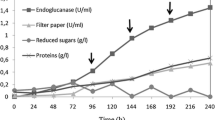
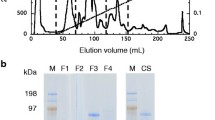
Abstract
Cellulomonas flavigena CDBB-531 was found to secrete a bifunctional cellulase/xylanase with a molecular mass of 49 kDa and pI 4.3. This enzyme was active on Remazol brilliant blue–carboxymethylcellulose (RBB-CMC) and Remazol brilliant blue–xylan (RBB-X). Based on thin-layer chromatographic analysis of the degradation products, the cellulase activity produced glucose, cellobiose, cellotriose, and cellotetraose from CMC as the substrate. When xylan from birchwood was used, end products were xylose, arabinose, and xylobiose. The bifunctional enzyme showed a pH optimum of 6 for cellulase activity and 9 for xylanase activity, which pointed out that this enzyme had separate sites for each activity. In both cases, the apparent optimum temperature was 50°C. The predicted amino acid sequence of purified protein showed similarity with the catalytic domain of several glycosyl hydrolases of family 10.


Similar content being viewed by others
References
Beg QK, Kapoor M, Mahajan L et al (2001) Microbial xylanases and their industrial application: a review. Appl Microbiol Biotechnol 56:326–338
Biely P, Mislovicová D, Toman R (1988) Remazol brilliant blue-xylan: a soluble chromogenic substrate for xylanases. Methods Enzymol 160:536–541
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Flint HJ, Martin J, McPherson CA et al (1993) A bifunctional enzyme, with separated xylanase and β (1,3-1,4)-glucanase domains, encoded by the xynD gene of Ruminococcus flavefaciens. J Bacteriol 175:2943–2951
Gilbert HJ, Hazlewood GP (1993) Bacterial cellulases and xylanases. J Gen Microbiol 139:187–194
Gutierrez-Nava A, Herrera-Herrera A, Mayorga-Reyes L et al (2003) Expression and characterization of the celcflB gene from Cellulomonas flavigena encoding an endo-β-1,4-glucanase. Curr Microbiol 47:359–363
Henrissat B, Bairoch A (1996) Updatind the sequence-based classification of glycosyl hydrolases. Biochem J 316:695–696
Kinter M, Sherman NE (2000) Protein sequencing and identification using tandem mass spectrometry. Wiley–Interscience, New York
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
MacLeod AM, Lindhoest T, Withers SG et al (1994) The acid/base catalyst in the exoglucanase/xylanase from Cellulomonas fimi is glutamic acid 127: evidence from detailed kinetic studies of mutants. Biochemistry 33:6371–6376
Martínez-Trujillo A, Pérez-Avalos O, Ponce-Noyola T (2003) Enzymatic properties of a purified xylanase from mutant PN-120 of Cellulomonas flavigena. Enzyme Microbiol Technol 32:401–406
Mayorga-Reyes L, Morales Y, Salgado LM et al (2002) Cellulomonas flavigena: characterization of an endo-1,4-xylanase tightly induced by sugar cane bagasse. FEMS Microbiol Lett 214:205–209
Natesh R, Bhanumoorthy P, Vithayathil PJ et al (1999) Crystal structure at 1.8 Å resolution and proposed amino acid sequence of a thermostable xylanase from Thermoascus aurantiacus. J Mol Biol 288:999–1012
Pérez-Avalos O, Ponce-Noyola T (2002) Synthesis and regulation of d-xylanase from Cellulomonas flavigena wild type and a mutant. Biotechnol Lett 24:813–817
Ponce-Noyola T, de la Torre M (1995) Isolation of a high-specific-growth-rate mutant of Cellulomonas flavigena on sugar cane bagasse. Appl Microbiol Biotechnol 45:709–712
Sambrook JE, Fritsch F, Maniatis T (1989) Molecular cloning: A laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
White A, Withers SG, Gilkes NR et al (1994) Crystal structure of the catalytic domain of the β-1,4-glycanase Cex from Cellulomonas fimi. Biochemistry 33:12546–12552
Wong KK, Tan LU, Saddler JN (1988) Multiplicity of β-1,4-xylanase in microorganisms: functions and applications. Microbiol Rev 52:305–317
Acknowledgments
This work was supported by Consejo Nacional de Ciencia y Tecnología-México (45678-Z). The authors thank the W. M. Keck Biomedical Mass Spectrometry Laboratory UVHS for sequencing the protein and Martha Mercado-Morales for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pérez-Avalos, O., Sánchez-Herrera, L.M., Salgado, L.M. et al. A Bifunctional Endoglucanase/Endoxylanase from Cellulomonas flavigena with Potential Use in Industrial Processes at Different pH. Curr Microbiol 57, 39–44 (2008). https://doi.org/10.1007/s00284-008-9149-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-008-9149-1