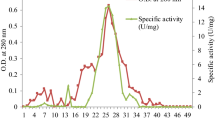
Abstract
EstA was purified from the supernatant by A. lwoffii 16C-1. Its molecular mass was determined to be 45 kDa, and the optimal activity occurred when the pH level was 8.0 at a temperature of 37°C. The activation energies for the hydrolysis of p-nitrophenyl butyrate was determined to be 11.25 kcal/mol in the temperature range of 10–37°C. The enzyme was unstable at temperatures higher than 50°C. The Michaelis constant (K m ) and V max for p-nitrophenyl butyrate were 11 μM and 131.6 μM min−1 mg of protein-1, respectively. The enzyme was strongly inhibited by Hg2−, Ca2+, Mg2+, Fe2+, Cu2+, Zn2+, Mn2+, Co2+, ethylemediaminetetraacetic acid (EDTA), phenylmethylsulfonyl fluoride (PMSF), and diisopropyl fluorophosphate (DFP).
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 20 August 2001 / Accepted: 20 September 2001
Rights and permissions
About this article
Cite this article
Kim, H., Park, K. Purification and Characterization of an Esterase from Acinetobacter lwoffii I6C-1. Curr Microbiol 44, 401–405 (2002). https://doi.org/10.1007/s00284-001-0008-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-001-0008-6