Abstract
The daily oscillations of bi ological and behavioural processes are controlled by the circadian clock circuitry that drives the physiology of the organism and, in particular, the functioning of the immune system in response to infectious agents. Circadian rhythmicity is known to affect both the pharmacokinetics and pharmacodynamics of pharmacological agents and vaccine-elicited immune responses. A better understanding of the role circadian pathways play in the regulation of virus replication will impact our clinical management of these diseases. This review summarises the experimental and clinical evidence on the interplay between different viral pathogens and our biological clocks, emphasising the importance of continuing research on the role played by the biological clock in virus-host organism interaction.
Similar content being viewed by others
Introduction
Almost all organisms are aware of the time of day and respond through an endogenous circadian clock, with an approximate cycle of 24 h. These clocks exist in virtually all tissues and are hierarchically organised. Upon sensing and integrating light signalling, the central pacemaker located in the suprachiasmatic nucleus (SCN) of the hypothalamus in the brain aligns its time to the external day/night cycles and provides neuronal and hormonal signals to synchronise the clocks in peripheral tissues [1]. At the cellular level, a molecular clock operates and sustains circadian oscillations in a wide range of cellular functions involving gene transcription, protein translation, intracellular signalling and metabolism, and tissue-specific functions [2]. Many aspects of our physiology show circadian rhythmicity, including host innate and adaptive immune response to infection or vaccination [3].
In mammals, the molecular clock is facilitated by a network of transcription factors and repressors that drive daily rhythmic gene expression. Circadian gene expression is generated by a transcriptional-translational feedback loop (TTFL) where the transcriptional activators CLOCK (circadian locomotor output cycles kaput) and BMAL1 (brain and muscle ARNT-like 1) bind to E-box motifs to drive the expression of the repressor protein period (PER1-3) and cryptochrome (CRY1-2), which inhibit CLOCK/BMAL1-dependent transcription. The transcription repressors REV-ERB α/β (reverse erythroblastosis virus alpha and beta) and activator RORα (retinoic acid-related [RAR] orphan receptor-alpha) provide an additional feedback loop to fine-tune the clock mechanism [2]. Recent studies report a disconnect between the rhythmic expression of mRNA and proteins [4, 5], highlighting a role for posttranscriptional and posttranslational pathways in defining protein activity in the circadian regulation of multiple cellular functions (Fig. 1). Over the last 5 years, several studies have shown a role for circadian pathways to influence viral infection by regulating a myriad of host factors that are essential for their replication [6, 7].
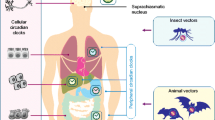
Host cell circadian rhythms and viral infection. A central master clock in the brain aligns sleep–wake and fasting-feeding cycles with the rotation of the Earth on its axis. The circadian clock exists in all tissues of the body, composing a network of timekeepers to anticipate rhythmic environmental changes. Cells have endogenous molecular clocks that operate autonomously, which enable them to keep track of time. In mammals, the molecular clock is orchestrated by several transcriptional-translational feedback loops. A disconnect between the rhythmic mRNA and proteins highlights a role for posttranscriptional and posttranslational pathways in defining protein activity in the circadian regulation of multiple cellular processes that are essential for viral replication. Created with BioRender.com
Viruses are obligate parasites, which rely on the host’s resources to replicate and spread. A typical viral life cycle starts with the engagement of the virus particle with a host factor(s), normally termed ‘viral receptors’ expressed on the surface of their target cells [8]. Following particle internalisation and disassembly of the viral capsid, the viral genome (RNA or DNA) is released into the cell to initiate the replicative cycle by exploiting the host transcriptional and translational machinery (Fig. 2). Given their absolute dependence on the host for replication, one may speculate that viruses have adapted to anticipate the rhythmic cellular environment and to exploit the predictability that circadian rhythms lend to our physiology. Whether this provides an evolutionary advantage likely depends on the viral strategy for persistence at the population level. For example, ‘hit-and-run’ viruses such as influenza A are best served by short replication cycles that produce new viruses at mucosal surfaces for onwards transmission. Whereas ‘life-long’ replication strategies seen with herpesviruses or hepatitis B virus need to maintain the reservoir of infected cells while evading or dampening host immune responses. One option for such persistent viruses is to couple their viral gene expression to the circadian TTFL.
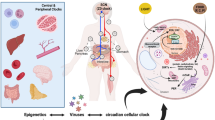
Virus replicative life cycle: viruses package their RNA or DNA genetic information within protein coats or capsids, and these particles engage with receptors at the cell surface that allow particle internalisation that primes capsid uncoating and release of genetic material. RNA viruses generate ‘replication factories’ within the cytoplasm that potentiate viral translation and assembly of new particles. The majority of DNA viruses replicate in the nucleus and after translation of the viral proteins and replication of the genome, new viral particles are assembled and released to complete the lifecycle. Created with BioRender.com
The impact of the circadian clock in regulating innate immunity against bacterial infections was more recently reported to influence adaptive immunity against viruses, an area, which has been extensively reviewed elsewhere [9,10,11,12,13,14,15,16]. Here, we summarise the findings of recent reports studying the interplay between viruses and our biological clocks at different tissue sites and discuss key areas for future research.
Viruses infecting the central nervous system and epidermis
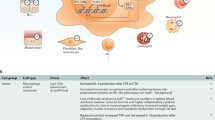
Herpes simplex virus (HSV) is a sexually transmitted pathogen that is prevalent worldwide. HSV-1 causes cold sores and HSV-2 causes most cases of genital herpes. Edgar et al. observed a significant enhancement of the replication of Murid Herpesvirus-4 (MuHV-4) and HSV-1 in BMAL1 knockout mice [17]. In wild-type animals, inoculation of MuHV-4 at the beginning of the resting phase resulted in a higher viral load compared to inoculation at the start of the active phase. The same study showed that MuHV-4 infection-induced BMAL1 expression regardless of the time of infection, suggesting that herpesviruses may influence or even override the host cellular clock [17]. Interestingly, HSV-1 was reported to infect the suprachiasmatic nuclei in CD4+ and CD8+ T-cell depleted mice, providing a potential mechanism for HSV to perturb the circadian clock [18]. Kalamvoki and Roizman showed that the HSV-encoded protein ICP0 interacts with the CLOCK:BMAL1 histone acetyltransferase complex and silencing of clock or expression of clock mutants reduced viral replication [19, 20], further evidence supporting the interaction of herpes viruses with circadian pathways. Matsuzawa et al. showed that HSV-2 infection was less severe in mice infected at the rest phase compared to the active phase and expression of the HSV-2 entry receptor Nectin1 (Pvrl1) is rhythmic and regulated by CLOCK binding to its promoter region [21]. Interestingly, the same study showed that the dose of acyclovir required to prevent HSV-2 infection was four times higher during the active phase compared to the resting phase [21].
Respiratory infections
Human lung diseases frequently show circadian variation in symptom severity and respiratory function and BMAL1 has been demonstrated to regulate respiratory inflammation [22]. Influenza A virus (IAV) is a leading cause of respiratory mortality and morbidity, and the role of circadian pathways in IAV infection was explored [23, 24] and reported that loss of BMAL1 in mice resulted in greater asthma-like airway changes and worse acute viral bronchitis. Furthermore, survival was higher when mice were infected before their active phase compared to the resting phase. Sengupta et al. did not observe differences in viral load when sampling infected mice at different time points. Infection at the onset of the active phase led to increased lung inflammation, independent of the viral burden, suggesting that more severe outcomes following influenza infection are mediated by time-of-day dependent regulation of host tolerance and immune activation pathways. A recent study reported that neonatal hyperoxia abolished the circadian-mediated time-of-day protection from IAV in mice [25]. Deletion of BMAL1 in alveolar type 2 (AT2) cells recapitulated the increase in IAV-associated mortality observed with the hyperoxia-exposed animals, demonstrating a role for the clock in alveolar type 2 cells to mediate the long-term effects of early-life exposure to the lung.
It is now widely recognised that the immune system is gated by the circadian clock [26], and a recent report demonstrated significant daytime variation in multiple immune parameters in > 300,000 participants in the UK Biobank, highlighting the diurnal nature of innate and adaptive immune responses [27]. Phillips et al. reported that vaccination in the morning induced greater antibody responses to both hepatitis A and influenza vaccines in human participants [28]. A subsequent larger randomised trial examined the time-of-day impact on the antibody response to the annual influenza vaccination in the elderly showed that morning vaccination (9–11 a.m.) increased viral specific antibody responses compared with afternoon vaccination (3–5 p.m.) [29]. These findings suggest that modulating the time of vaccination may provide a simple and practical measure to enhance vaccine efficacy and to provide greater protection. An additional influenza vaccination study reported that the time of sample collection rather than vaccination had a more significant effect on antibody responses [30].
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the causative agent of COVID-19, has affected millions of people to date. Cross-disciplinary approaches and collaborative efforts have led to an unprecedented speed in developing novel therapies and vaccines to tackle the COVID-19 pandemic. Mapping of the interactome of SARS-CoV-2 viral proteins identified 66 druggable host factors [31], and 30% of these host genes are rhythmic in the mouse liver [32], suggesting a potential circadian regulation of SARS-CoV-2 replication, and chronotherapies may be beneficial in treating COVID-19 [32, 33]. McNaughton et al. investigated more than 30,000 polymerase chain reaction (PCR) tests of nasopharyngeal swab samples and observed a twofold variation in the frequency of positive results across a 24-h period, with the peak of positivity in the early afternoon (around 2 p.m.) [34]. While evidence from other studies monitoring viral RNA at different times of day is required to consolidate this observation, it is important to remember these diagnostic assays measure SARS-CoV-2 RNA and not infectious virus [35] and future studies should assess the circadian dependency of infectious virus in the upper respiratory tract to interpret the impact on transmission rates.
Recently, Zhuang et al. reported a role for BMAL1 in modulating the susceptibility of lung epithelial cells to SARS-CoV-2 infection [36]. In this study, BMAL1 silencing resulted in a reduced expression of the viral receptor, angiotensin-converting enzyme 2 (ACE2), and less viral entry into lung epithelial cells. However, since the factors and mechanisms involved in SARS-CoV-2 entry are still being identified [37,38,39] and an extensive range of host pathways are known to be regulated by BMAL1, it is likely that further circadian-dependent or -independent pathways contribute to the SARS-CoV-2 entry beyond ACE2. The study further showed that silencing or pharmacological inhibition of BMAL1 induced transcription of a range of interferon-stimulated genes (ISGs) which possess broad antiviral activity [40]. An independent study showed time-of-day differences in ISG transcription following pharmacological activation of the type I IFN response in mouse skin models[41]. Whether baseline IFN expression and ISG protein levels in peripheral tissues exhibit a circadian rhythm remains to be established. Further research is needed to determine if the magnitude of host type I/III IFN responses varies depending on the time of day when cells encounter a virus and if this impacts the replication or disease progression.
Although SARS-CoV-2 primarily infects the upper and lower respiratory tract, several lines of evidence have demonstrated viral replication within intestinal epithelial cells in the gut [38, 42, 43]. Given the role of the gut microbiota in regulating immune function and the biological clock at both systemic and local levels [44,45,46], and that SARS-CoV-2 infection can perturb the gut microbiota [47], it is plausible that improving gut microbiota diversity by personalised nutrition and supplementation could be beneficial to reduce disease severity, especially in elderly and immune-compromised patients.
Vaccine development against SARS-CoV-2 has progressed at an unprecedented speed and several vaccines have been approved that have slowed the incidence of new infections and reduced disease severity [48, 49]. A recent observational study of > 2700 health care workers showed increased anti-Spike antibody levels in the afternoon compared to the morning in subjects receiving either mRNA or adenovirus-based vaccines [50]. This contrasts to an independent report of a small cohort of health care workers (n = 66) immunised with an inactivated SARS-CoV-2 vaccine, that showed increased anti-spike antibodies in participants vaccinated in the morning [51]. The differences between these two COVID-19 vaccine studies may reflect the different vaccine formulations where Zhang et al. studied an inactivated whole-virus immunogen that will likely induce polytypic responses to a range of SARS-CoV-2 encoded proteins. When comparing the effect of administration time on antibody responses elicited to IAV and SARS-CoV-2 vaccines we need to consider the cohorts under study, particularly with regard to immune status; where responses to influenza vaccine will involve the stimulation of memory responses, whereas the health care worker cohorts vaccinated in early 2021 will have involved seronegative participants. It is important to recognise the limitations of these vaccine studies where the sleep and shift-work patterns of the participants that are known to influence vaccine responses [52,53,54], were not available. Furthermore, neither cohort included children or high-risk groups, such as the elderly or immunocompromised. It is worth noting that a recent study of health care workers demonstrated that participants who perform shift work are associated with positive COVID-19 tests compared with those who do not perform shift work [55]. Additional studies are warranted to evaluate the circadian regulation of natural and vaccine-induced SARS-CoV-2 immunity.
Hepatotropic infections
Hepatitis B virus (HBV) is a globally important pathogen with over 270 million people chronically infected worldwide and a leading cause of cirrhosis and hepatocellular carcinoma (HCC) [56]. Current treatments only suppress viral replication and are not curative due to the persistence of viral DNA in hepatocytes. Approximately 20% of genes in the liver are expressed in a circadian pattern [57], suggesting that the virus has evolved to persist and to cope with rhythmic metabolic changes in the liver. A recent study reported that the viral receptor that is essential for HBV entry (sodium taurocholate cotransporting polypeptide—NTCP) displayed a circadian pattern in synchronised human hepatocytes. Importantly, BMAL1 activated HBV transcription via direct binding to the viral genome. Pharmacological inhibition of BMAL1 using REV-ERB ligands inhibited HBV transcription and production of viral antigens in vitro and in vivo in a human liver chimeric mouse model [58]. Interestingly, multiple E-box motifs are conserved among members of the Hepadnaviridae family, consistent with an evolutionarily conserved role for the circadian pathway in regulating this family of small DNA viruses.
Given the breadth of processes under circadian control, viruses could efficiently induce a cellular environment more conducive to replication by targeting the circadian clock. An early study reported that overexpression of the HBV regulatory X protein (HBx) perturbed core circadian gene expression [59]. Furthermore, several core clock gene transcripts were perturbed (reduced BMAL1 and increased REV-ERBs) in the chronic hepatitis B liver compared with uninfected individuals [58]. Since several studies have reported an association of HCC with disrupted circadian expression [60, 61], it is tempting to speculate a causative relationship between HBV-specific clock perturbation and its pathogenicity which warrants further investigations.
In contrast to HBV, HCV is an RNA virus, which replicates in the cytoplasm of human hepatocytes; consequently, its interplay with the hepatic clock will differ from HBV. An interesting clinical observation in HCV patients with end-stage liver failure receiving a liver transplant showed that the viral rebound was faster when the surgery was performed in the morning compared to the afternoon [62], suggesting a time-of-day dependency of HCV replication. A further study reported that BMAL1 and REV-ERBα influenced several steps in the HCV life cycle, including viral particle entry into hepatocytes and RNA genome replication. Deletion of BMAL1 by CRISPR knockout and overexpression or activation of REV-ERB with synthetic agonists inhibited the replication of HCV and the related flaviviruses dengue and Zika, which share the same lipid signalling pathways for their replication [63]. Benegiamo et al. showed that HCV core protein expression reduced PER2 and CRY2 protein level in vitro models [64]. Since perturbation of circadian pathways in the liver may contribute to liver disease [65], further studies using a replicative virus and in vivo models would be necessary to conclude an HCV-induced circadian perturbation.
Viruses infecting the immune system
Human immunodeficiency virus 1 (HIV-1) is a life-threatening pathogen that lacks either a curative therapy or vaccine. In HIV-infected individuals on suppressive antiretroviral therapy (ART), the viral genome persists in long-lived latently infected CD4+ T-cells [66]. An association between peripheral viral RNA levels in HIV-infected individuals on ART and the time of day of sampling has been demonstrated and BMAL1 expression was associated with an increased level of unspliced viral RNAs [67, 68]. In the same study, a BMAL1 binding E-box motif was identified in the long terminal repeat (LTR) of the viral genome and ectopic coexpression of BMAL1 and CLOCK enhanced LTR activity, this phenotype was lost when the E-box was mutated [67]. An independent study demonstrated that genetic silencing of the transcription repressor REV-ERB increases HIV-LTR activity and that pharmacological activation of REV-ERB, which represses BMAL1, reduced viral replication in primary CD4+ T-cells and macrophages [69]. Interestingly, motif analysis of the HIV-1 LTR uncovered additional circadian regulatory elements including the RORE (ROR response element) and the glucocorticoid response element across diverse HIV subtypes. Since some of these clock motifs overlap with the binding sites of other host factors, which also drive HIV-1 transcription, it is tempting to speculate that the clock components may perform ‘shiftwork’ in place of other host transcription factors to drive rhythmic HIV transcription. In contrast, the HIV-encoded gene viral protein transactivator of transcription (Tat) has been shown to reset the circadian rhythm in vitro and in vivo at clinically relevant concentrations through the N-methyl-d-aspartate receptor pathway [70]. The role of Tat protein in regulating the circadian rhythm was further demonstrated in an inducible Tat transgenic mouse system, where a decrease of circadian wheel-running and locomotor activity was observed compared with control mice [71].
Conclusions and future directions
All viruses must co-opt the host cell translational machinery to synthesise proteins required for new particle assembly. Under physiological conditions, the cellular proteome is coordinated by mammalian target-of-rapamycin complexes (mTORC), which undergo posttranslational modification to balance protein synthesis and degradation to maintain homeostasis [5]. Recent reports showing that BMAL1, PER, and CRY modulate mTORC activity [72, 73] and their genetic ablation perturbs protein homeostasis [74, 75], highlights a role for this pathway in the rhythmic expression of proteasomal activity and biosynthetic resources such as ATP and amino acid availability [76,77,78,79,80]. It is interesting to note that many viruses subvert mTORC signalling to promote and sustain viral protein synthesis [81]; however, the influence of endogenous oscillations in mTORC activity and stress responses during infection remains to be explored.
Circadian factors and timings have been shown to modulate every aspect of life and it is unsurprising that this is true for viral infections. The emerging picture of time dependence in the replication of almost all viruses studied to date, whether they are DNA or RNA based and mediate acute or chronic infection, suggests that the circadian influences on infection are ubiquitous. The diurnal variation observed in drug sensitivity suggests opportunities to apply a ‘chronotherapeutic’ approach to optimise antiviral dosing. Similarly monitoring the impact of time of day on antiviral antibody responses could lead to simple improvements in vaccine efficacy.
References
Astiz M, Heyde I, Oster H (2019) Mechanisms of Communication in the Mammalian Circadian Timing System. Int J Mol Sci 20(2)
Takahashi JS (2017) Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet 18(3):164–179
Allada R, Bass J (2021) Circadian mechanisms in medicine. N Engl J Med 384(6):550–561
Stangherlin A, Seinkmane E, O’Neill JS (2021) Understanding circadian regulation of mammalian cell function, protein homeostasis, and metabolism. Curr Opin Syst Biol 28
Wong DC, O’Neill JS (2018) Non-transcriptional processes in circadian rhythm generation. Curr Opin Physiol 5:117–132
Zhuang X et al (2017) Interplay between circadian clock and viral infection. J Mol Med (Berl) 95(12):1283–1289
Borrmann H, McKeating JA, Zhuang X (2021) The circadian clock and viral infections. J Biol Rhythms 36(1):9–22
Helenius A (2018) Virus entry: looking back and moving forward. J Mol Biol 430(13):1853–1862
Palomino-Segura M, Hidalgo A (2021) Circadian immune circuits. J Exp Med 218(2)
Hergenhan S, Holtkamp S, Scheiermann C (2020) Molecular interactions between components of the circadian clock and the immune system. J Mol Biol 432(12):3700–3713
Cermakian N et al (2021) Circadian rhythms in adaptive immunity and vaccination. Semin Immunopathol
Downton P, Early JO, Gibbs JE (2020) Circadian rhythms in adaptive immunity. Immunology 161(4):268–277
Kinouchi K et al (2021) Circadian rhythms in the tissue-specificity from metabolism to immunity: insights from omics studies. Mol Aspects Med 80:100984
Waggoner SN (2020) Circadian rhythms in immunity. Curr Allergy Asthma Rep 20(1):2
Haspel JA et al (2020) Perfect timing: circadian rhythms, sleep, and immunity - an NIH workshop summary. JCI Insight 5(1)
Ikuta K, Scheiermann C (2020) Editorial: circadian control of immunity. Front Immunol 11:618843
Edgar RS et al (2016) Cell autonomous regulation of herpes and influenza virus infection by the circadian clock. Proc Natl Acad Sci U S A 113(36):10085–10090
Matsubara S, Atherton SS (1997) Spread of HSV-1 to the suprachiasmatic nuclei and retina in T cell depleted BALB/c mice. J Neuroimmunol 80(1–2):165–171
Kalamvoki M, Roizman B (2011) The histone acetyltransferase CLOCK is an essential component of the herpes simplex virus 1 transcriptome that includes TFIID, ICP4, ICP27, and ICP22. J Virol 85(18):9472–9477
Kalamvoki M, Roizman B (2010) Circadian CLOCK histone acetyl transferase localizes at ND10 nuclear bodies and enables herpes simplex virus gene expression. Proc Natl Acad Sci U S A 107(41):17721–17726
Matsuzawa T et al (2018) Differential day-night outcome to HSV-2 cutaneous infection. J Invest Dermatol 138(1):233–236
Ince LM et al (2019) Circadian variation in pulmonary inflammatory responses is independent of rhythmic glucocorticoid signaling in airway epithelial cells. FASEB J 33(1):126–139
Sengupta S et al (2019) Circadian control of lung inflammation in influenza infection. Nat Commun 10(1):4107
Ehlers A et al (2018) BMAL1 links the circadian clock to viral airway pathology and asthma phenotypes. Mucosal Immunol 11(1):97–111
Issah Y et al (2021) Loss of circadian protection against influenza infection in adult mice exposed to hyperoxia as neonates. Elife 10
Scheiermann C et al (2018) Clocking in to immunity. Nat Rev Immunol 18(7):423–437
Wyse C et al (2021) Seasonal and daytime variation in multiple immune parameters in humans: evidence from 329,261 participants of the UK Biobank cohort. iScience 24(4):102255
Phillips AC et al (2008) Preliminary evidence that morning vaccination is associated with an enhanced antibody response in men. Psychophysiology 45(4):663–666
Long JE et al (2016) Morning vaccination enhances antibody response over afternoon vaccination: a cluster-randomised trial. Vaccine 34(24):2679–2685
Kurupati RK et al (2017) The effect of timing of influenza vaccination and sample collection on antibody titers and responses in the aged. Vaccine 35(30):3700–3708
Gordon DE et al (2020) A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583(7816):459–468
Ray S, Reddy AB (2020) COVID-19 management in light of the circadian clock. Nat Rev Mol Cell Biol 21(9):494–495
Meira ECM, Miyazawa M, Gozal D (2020) Putative contributions of circadian clock and sleep in the context of SARS-CoV-2 infection. Eur Respir J 55(6)
McNaughton CD, A.N., Johnson CH, Ward MJ, Lasko TA., Diurnal variation in SARS-CoV-2 PCR test results: test accuracy may vary by time of day. medRxiv, 2021 Jan 1.
Michalakis Y et al (2021) SARS-CoV-2 viral RNA levels are not “viral load.” Trends Microbiol 29(11):970–972
Zhuang X et al (2021) The circadian clock component BMAL1 regulates SARS-CoV-2 entry and replication in lung epithelial cells. iScience 24(10):103144
Cantuti-Castelvetri L et al (2020) Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 370(6518):856–860
Sungnak W et al (2020) SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 26(5):681–687
Shang J et al (2020) Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A 117(21):11727–11734
Schoggins JW, Rice CM (2011) Interferon-stimulated genes and their antiviral effector functions. Curr Opin Virol 1(6):519–525
Greenberg EN et al (2020) Circadian control of interferon-sensitive gene expression in murine skin. Proc Natl Acad Sci U S A 117(11):5761–5771
Qian Q et al (2021) Direct evidence of active SARS-CoV-2 replication in the intestine. Clin Infect Dis 73(3):361–366
Stanifer ML et al (2020) Critical role of type III interferon in controlling SARS-CoV-2 infection in human intestinal epithelial cells. Cell Rep 32(1):107863
Tuganbaev T et al (2020) Diet diurnally regulates small intestinal microbiome-epithelial-immune homeostasis and enteritis. Cell 182(6):1441-1459 e21
Choi H, Rao MC, Chang EB (2021) Gut microbiota as a transducer of dietary cues to regulate host circadian rhythms and metabolism. Nat Rev Gastroenterol Hepatol 18(10):679–689
Brooks JF 2nd et al (2021) The microbiota coordinates diurnal rhythms in innate immunity with the circadian clock. Cell 184(16):4154-4167 e12
Zuo T et al (2020) Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology 159(3):944-955 e8
Bakhiet M, Taurin S (2021) SARS-CoV-2: targeted managements and vaccine development. Cytokine Growth Factor Rev 58:16–29
Xia S et al (2021) Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis 21(1):39–51
Wang W et al (2021) Time of day of vaccination affects SARS-CoV-2 antibody responses in an observational study of healthcare workers. J Biol Rhythms. in press
Zhang H et al (2021) Time of day influences immune response to an inactivated vaccine against SARS-CoV-2. Cell Res
Prather AA et al (2021) Temporal links between self-reported sleep and antibody responses to the influenza vaccine. Int J Behav Med 28(1):151–158
Lange T et al (2003) Sleep enhances the human antibody response to hepatitis A vaccination. Psychosom Med 65(5):831–835
Spiegel K, Sheridan JF, Van Cauter E (2002) Effect of sleep deprivation on response to immunization. JAMA 288(12):1471–1472
Maidstone R et al (2021) Shift work is associated with positive COVID-19 status in hospitalised patients. Thorax 76(6):601–606
Global hepatitis Report WHO, 2017
Zhang R et al (2014) A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A 111(45):16219–16224
Zhuang X et al (2021) Circadian control of hepatitis B virus replication. Nat Commun 12(1):1658
Yang SL et al (2014) Hepatitis B virus X protein disrupts the balance of the expression of circadian rhythm genes in hepatocellular carcinoma. Oncol Lett 8(6):2715–2720
Lin YM et al (2008) Disturbance of circadian gene expression in hepatocellular carcinoma. Mol Carcinog 47(12):925–933
VoPham T et al (2018) Circadian misalignment and hepatocellular carcinoma incidence in the United States. Cancer Epidemiol Biomarkers Prev 27(7):719–727
Zhuang X et al (2018) Daytime variation in hepatitis C virus replication kinetics following liver transplant. Wellcome Open Res 3:96
Zhuang X et al (2019) The circadian clock components BMAL1 and REV-ERBalpha regulate flavivirus replication. Nat Commun 10(1):377
Benegiamo G et al (2013) Mutual antagonism between circadian protein period 2 and hepatitis C virus replication in hepatocytes. PLoS One 8(4):e60527
Mukherji A et al (2019) The circadian clock and liver function in health and disease. J Hepatol 71(1):200–211
Chun TW et al (1997) Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387(6629):183–188
Chang CC et al (2018) Variation in cell-associated unspliced HIV RNA on antiretroviral therapy is associated with the circadian regulator brain-and-muscle-ARNT-like-1. AIDS 32(15):2119–2128
Stern J et al (2021) Cell-associated HIV RNA has a circadian cycle in males living with HIV on antiretroviral therapy. J Infect Dis
Borrmann H et al (2020) Pharmacological activation of the circadian component REV-ERB inhibits HIV-1 replication. Sci Rep 10(1):13271
Clark JP 3rd et al (2005) HIV protein, transactivator of transcription, alters circadian rhythms through the light entrainment pathway. Am J Physiol Regul Integr Comp Physiol 289(3):R656–R662
Duncan MJ et al (2008) Effects of chronic expression of the HIV-induced protein, transactivator of transcription, on circadian activity rhythms in mice, with or without morphine. Am J Physiol Regul Integr Comp Physiol 295(5):R1680–R1687
Lipton JO et al (2015) The circadian protein BMAL1 regulates translation in response to S6K1-mediated phosphorylation. Cell 161(5):1138–1151
Wu R et al (2019) The circadian protein Period2 suppresses mTORC1 activity via recruiting Tsc1 to mTORC1 complex. Cell Metab 29(3):653-667 e6
Lipton JO et al (2017) Aberrant proteostasis of BMAL1 underlies circadian abnormalities in a paradigmatic mTOR-opathy. Cell Rep 20(4):868–880
Putker M et al (2021) Cryptochromes confer robustness, not rhythmicity, to circadian timekeeping. EMBO J 40(7):e106745
Krishnaiah SY et al (2017) Clock regulation of metabolites reveals coupling between transcription and metabolism. Cell Metab 25(4):961-974 e4
Feeney KA et al (2016) Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature 532(7599):375–379
Luck S et al (2014) Rhythmic degradation explains and unifies circadian transcriptome and proteome data. Cell Rep 9(2):741–751
Ryzhikov M et al (2019) Diurnal rhythms spatially and temporally organize autophagy. Cell Rep 26(7):1880-1892 e6
Castelo-Szekely V, Gatfield D (2020) Emerging roles of translational control in circadian timekeeping. J Mol Biol 432(12):3483–3497
Le Sage V et al (2016) Adapting the stress response: viral subversion of the mTOR signaling pathway. Viruses 8(6)
Acknowledgements
We thank Helene Borrmann for many exciting discussions about viruses and the clock for providing comments on this review. We thank John O’Neill and Christine Styles for the helpful discussion. The McKeating laboratory is funded by a Wellcome Investigator Award 200838/Z/16/Z, UK Medical Research Council (MRC) project grant MR/R022011/1 and Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Science (CIFMS), China (grant number: 2018-I2M-2-002). The Edgar Lab is funded by a Wellcome Trust-Royal Society Sir Henry Dale Fellowship (208790/Z/17/Z).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is a contribution to the special issue on: Chronoimmunology: from preclinical assessments to clinical applications - Guest Editors: Henrik Oster & David Ray
Rights and permissions
About this article
Cite this article
Zhuang, X., Edgar, R.S. & McKeating, J.A. The role of circadian clock pathways in viral replication. Semin Immunopathol 44, 175–182 (2022). https://doi.org/10.1007/s00281-021-00908-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-021-00908-2