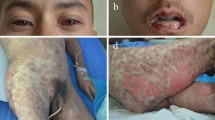
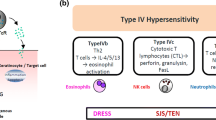
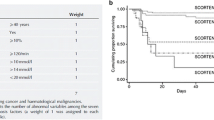
Abstract
Adverse cutaneous drug reactions are recognized as being major health problems worldwide causing considerable costs for health care systems. Most adverse cutaneous drug reactions follow a benign course; however, up to 2 % of all adverse cutaneous drug eruptions are severe and life-threatening. These include acute generalized exanthematous pustulosis (AGEP), drug reaction with eosinophilia and systemic symptoms (DRESS), Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN). Physicians should be aware of specific red flags to rapidly identify these severe cutaneous drug eruptions and initiate appropriate treatment. Besides significant progress in clinical classification and treatment, recent studies have greatly enhanced our understanding in the pathophysiology of adverse cutaneous drug reactions. Genetic susceptibilities to certain drugs have been identified in SJS/TEN patients, viral reactivation in DRESS has been elucidated, and the discovery of tissue resident memory T cells helps to better understand the recurrent site-specific inflammation in patients with fixed drug eruption.






Similar content being viewed by others
References
French LE, Prins C (2013) Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis. In: Bolognia JL, Jorrizo JL, Schaffer JV (eds) Dermatology, vol 3, 3rd edn. Elsevier, New York, pp 319–334
Bircher AJ, Scherer K (2010) Delayed cutaneous manifestations of drug hypersensitivity. Med Clin North Am 94(4):711–725. doi:10.1016/j.mcna.2010.04.001
Brockow K, Garvey LH, Aberer W, Atanaskovic-Markovic M, Barbaud A, Bilo MB, Bircher A, Blanca M, Bonadonna B, Campi P, Castro E, Cernadas JR, Chiriac AM, Demoly P, Grosber M, Gooi J, Lombardo C, Mertes PM, Mosbech H, Nasser S, Pagani M, Ring J, Romano A, Scherer K, Schnyder B, Testi S, Torres M, Trautmann A, Terreehorst I, Group EEDAI (2013) Skin test concentrations for systemically administered drugs—an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy 68(6):702–712. doi:10.1111/all.12142
Hoffmann HJ, Santos AF, Mayorga C, Nopp A, Eberlein B, Ferrer M, Rouzaire P, Ebo D, Sabato V, Sanz ML, Pecaric-Petkovic T, Patil SU, Hausmann OV, Shreffler WG, Korosec P, Knol EF (2015) The clinical utility of basophil activation testing in diagnosis and monitoring of allergic disease. Allergy. doi:10.1111/all.12698
Sicherer SH, Leung DY (2015) Advances in allergic skin disease, anaphylaxis, and hypersensitivity reactions to foods, drugs, and insects in 2014. J Allergy Clin Immunol 135(2):357–367. doi:10.1016/j.jaci.2014.12.1906
Porebski G, Pecaric-Petkovic T, Groux-Keller M, Bosak M, Kawabata TT, Pichler WJ (2013) In vitro drug causality assessment in Stevens-Johnson syndrome—alternatives for lymphocyte transformation test. Clin Exp Allergy 43(9):1027–1037. doi:10.1111/cea.12145
Polak ME, Belgi G, McGuire C, Pickard C, Healy E, Friedmann PS, Ardern-Jones MR (2013) In vitro diagnostic assays are effective during the acute phase of delayed-type drug hypersensitivity reactions. Br J Dermatol 168(3):539–549. doi:10.1111/bjd.12109
Rozieres A, Hennino A, Rodet K, Gutowski MC, Gunera-Saad N, Berard F, Cozon G, Bienvenu J, Nicolas JF (2009) Detection and quantification of drug-specific T cells in penicillin allergy. Allergy 64(4):534–542. doi:10.1111/j.1398-9995.2008.01674.x
Demoly P, Adkinson NF, Brockow K, Castells M, Chiriac AM, Greenberger PA, Khan DA, Lang DM, Park HS, Pichler W, Sanchez-Borges M, Shiohara T, Thong BY (2014) International consensus on drug allergy. Allergy 69(4):420–437. doi:10.1111/all.12350
Bigby M, Jick S, Jick H, Arndt K (1986) Drug-induced cutaneous reactions. A report from the Boston Collaborative Drug Surveillance Program on 15,438 consecutive inpatients, 1975 to 1982. Jama 256(24):3358–3363
Bigby M (2001) Rates of cutaneous reactions to drugs. Arch Dermatol 137(6):765–770
Nigen S, Knowles SR, Shear NH (2003) Drug eruptions: approaching the diagnosis of drug-induced skin diseases. J Drugs Dermatol: JDD 2(3):278–299
Hausermann P, Harr T, Bircher AJ (2004) Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis 51(5-6):297–310. doi:10.1111/j.0105-1873.2004.00445.x
Gell PGH, Coombs RRA (1963) Clinical aspects of immunology. Blackwell, Oxford
Scherer K, Spoerl D, Bircher AJ (2010) Adverse drug reactions to biologics. J Dtsch Dermatol Gesellschaft = J German Soc Dermatol: JDDG 8(6):411–426. doi:10.1111/j.1610-0387.2010.07339.x
Tohyama M, Hashimoto K, Yasukawa M, Kimura H, Horikawa T, Nakajima K, Urano Y, Matsumoto K, Iijima M, Shear NH (2007) Association of human herpesvirus 6 reactivation with the flaring and severity of drug-induced hypersensitivity syndrome. Br J Dermatol 157(5):934–940. doi:10.1111/j.1365-2133.2007.08167.x
Campos-Fernandez Mdel M, Ponce-De-Leon-Rosales S, Archer-Dubon C, Orozco-Topete R (2005) Incidence and risk factors for cutaneous adverse drug reactions in an intensive care unit. Revista Investig Clin; Organo Hosp Enferm Nutr 57(6):770–774
Schnyder B, Brockow K (2015) Pathogenesis of drug allergy, current concepts and recent insights. Clin Exp Allergy. doi:10.1111/cea.12591
Korkij W, Soltani K (1984) Fixed drug eruption. A brief review. Arch Dermatol 120(4):520–524
Shiohara T (2009) Fixed drug eruption: pathogenesis and diagnostic tests. Curr Opin Allergy Clin Immunol 9(4):316–321. doi:10.1097/ACI.0b013e32832cda4c
Mizukawa Y, Yamazaki Y, Teraki Y, Hayakawa J, Hayakawa K, Nuriya H, Kohara M, Shiohara T (2002) Direct evidence for interferon-gamma production by effector-memory-type intraepidermal T cells residing at an effector site of immunopathology in fixed drug eruption. Am J Pathol 161(4):1337–1347
Mizukawa Y, Shiohara T (2010) Nonpigmenting fixed drug eruption as a possible abortive variant of toxic epidermal necrolysis: immunohistochemical and serum cytokine analyses. Clin Exp Dermatol 35(5):493–497. doi:10.1111/j.1365-2230.2009.03622.x
Mizukawa Y, Yamazaki Y, Shiohara T (2008) In vivo dynamics of intraepidermal CD8+ T cells and CD4+ T cells during the evolution of fixed drug eruption. Br J Dermatol 158(6):1230–1238. doi:10.1111/j.1365-2133.2008.08516.x
Park CO, Kupper TS (2015) The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med 21(7):688–697. doi:10.1038/nm.3883
Cheuk S, Wiken M, Blomqvist L, Nylen S, Talme T, Stahle M, Eidsmo L (2014) Epidermal Th22 and Tc17 cells form a localized disease memory in clinically healed psoriasis. J Immunol 192(7):3111–3120. doi:10.4049/jimmunol.1302313
Watanabe R, Gehad A, Yang C, Scott LL, Teague JE, Schlapbach C, Elco CP, Huang V, Matos TR, Kupper TS, Clark RA (2015) Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci Trans Med 7(279):279ra239. doi:10.1126/scitranslmed.3010302
Shiohara T, Ushigome Y, Kano Y, Takahashi R (2014) Crucial role of viral reactivation in the development of severe drug eruptions: a comprehensive review. Clin Rev Allergy Immunol. doi:10.1007/s12016-014-8421-3
Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS (2012) Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature 483(7388):227–231. doi:10.1038/nature10851
Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS (2010) Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat Med 16(2):224–227. doi:10.1038/nm.2078
Liu L, Fuhlbrigge RC, Karibian K, Tian T, Kupper TS (2006) Dynamic programming of CD8+ T cell trafficking after live viral immunization. Immunity 25(3):511–520. doi:10.1016/j.immuni.2006.06.019
Lyell A (1967) A review of toxic epidermal necrolysis in Britain. Br J Dermatol 79(12):662–671
Rzany B, Correia O, Kelly JP, Naldi L, Auquier A, Stern R (1999) Risk of Stevens-Johnson syndrome and toxic epidermal necrolysis during first weeks of antiepileptic therapy: a case-control study. Study Group of the International Case Control Study on Severe Cutaneous Adverse Reactions. Lancet 353(9171):2190–2194
La Grenade L, Lee L, Weaver J, Bonnel R, Karwoski C, Governale L, Brinker A (2005) Comparison of reporting of Stevens-Johnson syndrome and toxic epidermal necrolysis in association with selective COX-2 inhibitors. Drug Saf 28(10):917–924
Aguiar D, Pazo R, Duran I, Terrasa J, Arrivi A, Manzano H, Martin J, Rifa J (2004) Toxic epidermal necrolysis in patients receiving anticonvulsants and cranial irradiation: a risk to consider. J Neuro-oncol 66(3):345–350
Aydin F, Cokluk C, Senturk N, Aydin K, Canturk MT, Turanli AY (2006) Stevens-Johnson syndrome in two patients treated with cranial irradiation and phenytoin. J Eur Acad Dermatol Venereol:JEADV 20(5):588–590. doi:10.1111/j.1468-3083.2006.01510.x
Lebargy F, Wolkenstein P, Gisselbrecht M, Lange F, Fleury-Feith J, Delclaux C, Roupie E, Revuz J, Roujeau JC (1997) Pulmonary complications in toxic epidermal necrolysis: a prospective clinical study. Intensive Care Med 23(12):1237–1244
Revuz J, Penso D, Roujeau JC, Guillaume JC, Payne CR, Wechsler J, Touraine R (1987) Toxic epidermal necrolysis. Clinical findings and prognosis factors in 87 patients. Arch Dermatol 123(9):1160–1165
Chang YS, Huang FC, Tseng SH, Hsu CK, Ho CL, Sheu HM (2007) Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis: acute ocular manifestations, causes, and management. Cornea 26(2):123–129. doi:10.1097/ICO.0b013e31802eb264
Sotozono C, Ueta M, Koizumi N, Inatomi T, Shirakata Y, Ikezawa Z, Hashimoto K, Kinoshita S (2009) Diagnosis and treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis with ocular complications. Ophthalmology 116(4):685–690. doi:10.1016/j.ophtha.2008.12.048
Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau J-C (1993) Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol 129:92–96
Yip LW, Thong BY, Lim J, Tan AW, Wong HB, Handa S, Heng WJ (2007) Ocular manifestations and complications of Stevens-Johnson syndrome and toxic epidermal necrolysis: an Asian series. Allergy 62(5):527–531. doi:10.1111/j.1398-9995.2006.01295.x
Magina S, Lisboa C, Leal V, Palmares J, Mesquita-Guimaraes J (2003) Dermatological and ophthalmological sequels in toxic epidermal necrolysis. Dermatology 207(1):33–36
Bentele-Jaberg N, Guenova E, Mehra T, Nageli M, Chang YT, Cozzio A, French LE, Hoetzenecker W (2015) The phytotherapeutic fenugreek as trigger of toxic epidermal necrolysis. Dermatology. doi:10.1159/000433423
Spielberg SP, Gordon GB, Blake DA, Goldstein DA, Herlong HF (1981) Predisposition to phenytoin hepatotoxicity assessed in vitro. N Engl J Med 305(13):722–727. doi:10.1056/NEJM198109243051302
Shear NH, Spielberg SP, Grant DM, Tang BK, Kalow W (1986) Differences in metabolism of sulfonamides predisposing to idiosyncratic toxicity. Ann Intern Med 105(2):179–184
Wolkenstein P, Carriere V, Charue D, Bastuji-Garin S, Revuz J, Roujeau JC, Beaune P, Bagot M (1995) A slow acetylator genotype is a risk factor for sulphonamide-induced toxic epidermal necrolysis and Stevens-Johnson syndrome. Pharmacogenetics 5(4):255–258
Dietrich A, Kawakubo Y, Rzany B, Mockenhaupt M, Simon JC, Schopf E (1995) Low N-acetylating capacity in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. Exp Dermatol 4(5):313–316
Yun J, Marcaida MJ, Eriksson KK, Jamin H, Fontana S, Pichler WJ, Yerly D (2014) Oxypurinol directly and immediately activates the drug-specific T cells via the preferential use of HLA-B*58:01. J Immunol 192(7):2984–2993. doi:10.4049/jimmunol.1302306
Illing PT, Vivian JP, Dudek NL, Kostenko L, Chen Z, Bharadwaj M, Miles JJ, Kjer-Nielsen L, Gras S, Williamson NA, Burrows SR, Purcell AW, Rossjohn J, McCluskey J (2012) Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature 486(7404):554–558. doi:10.1038/nature11147
Ostrov DA, Grant BJ, Pompeu YA, Sidney J, Harndahl M, Southwood S, Oseroff C, Lu S, Jakoncic J, de Oliveira CA, Yang L, Mei H, Shi L, Shabanowitz J, English AM, Wriston A, Lucas A, Phillips E, Mallal S, Grey HM, Sette A, Hunt DF, Buus S, Peters B (2012) Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire. Proc Natl Acad Sci U S A 109(25):9959–9964. doi:10.1073/pnas.1207934109
Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP, Lin YL, Lan JL, Yang LC, Hong HS, Chen MJ, Lai PC, Wu MS, Chu CY, Wang KH, Chen CH, Fann CS, Wu JY, Chen YT (2005) HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A 102(11):4134–4139
Chung WH, Hung SI, Chen YT (2007) Human leukocyte antigens and drug hypersensitivity. Curr Opin Allergy Clin Immunol 7(4):317–323. doi:10.1097/ACI.0b013e3282370c5f
Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, Wu JY, Chen YT (2004) Medical genetics: a marker for Stevens-Johnson syndrome. Nature 428(6982):486
Chen P, Lin JJ, Lu CS, Ong CT, Hsieh PF, Yang CC, Tai CT, Wu SL, Lu CH, Hsu YC, Yu HY, Ro LS, Lu CT, Chu CC, Tsai JJ, Su YH, Lan SH, Sung SF, Lin SY, Chuang HP, Huang LC, Chen YJ, Tsai PJ, Liao HT, Lin YH, Chen CH, Chung WH, Hung SI, Wu JY, Chang CF, Chen L, Chen YT, Shen CY, Taiwan SJSC (2011) Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med 364(12):1126–1133. doi:10.1056/NEJMoa1009717
Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, Hunziker T, Saurat JH, Tschopp J, French LE (1998) Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science 282(5388):490–493
Wehrli P, Viard I, Bullani R, Tschopp J, French LE (2000) Death receptors in cutaneous biology and disease. J Investig Dermatol 115(2):141–148. doi:10.1046/j.1523-1747.2000.00037.x
Ito K, Hara H, Okada T, Shimojima H, Suzuki H (2004) Toxic epidermal necrolysis treated with low-dose intravenous immunoglobulin: immunohistochemical study of Fas and Fas-ligand expression. Clin Exp Dermatol 29(6):679–680
Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, Chin SW, Chiou CC, Chu SC, Ho HC, Yang CH, Lu CF, Wu JY, Liao YD, Chen YT (2008) Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med 14(12):1343–1350. doi:10.1038/nm.1884
Saito N, Qiao H, Yanagi T, Shinkuma S, Nishimura K, Suto A, Fujita Y, Suzuki S, Nomura T, Nakamura H, Nagao K, Obuse C, Shimizu H, Abe R (2014) An annexin A1-FPR1 interaction contributes to necroptosis of keratinocytes in severe cutaneous adverse drug reactions. Sci Transl Med 6(245):245
Viard-Leveugle I, Bullani RR, Meda P, Micheau O, Limat A, Saurat JH, Tschopp J, French LE (2003) Intracellular localization of keratinocyte Fas ligand explains lack of cytolytic activity under physiological conditions. J Biol Chem 278(18):16183–16188
Teraki Y, Shiohara T (2003) IFN-gamma-producing effector CD8+ T cells and IL-10-producing regulatory CD4+ T cells in fixed drug eruption. J Allergy Clin Immunol 112(3):609–615
Teraki Y, Kawabe M, Izaki S (2013) Possible role of TH17 cells in the pathogenesis of Stevens-Johnson syndrome and toxic epidermal necrolysis. J Allergy Clin Immunol 131(3):907–909. doi:10.1016/j.jaci.2012.08.042
Gagliani N, Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, de Zoete MR, Licona-Limon P, Paiva RS, Ching T, Weaver C, Zi X, Pan X, Fan R, Garmire LX, Cotton MJ, Drier Y, Bernstein B, Geginat J, Stockinger B, Esplugues E, Huber S, Flavell RA (2015) Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 523(7559):221–225. doi:10.1038/nature14452
Ang CC, Tay YK (2011) Hematological abnormalities and the use of granulocyte-colony-stimulating factor in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. Int J Dermatol 50(12):1570–1578. doi:10.1111/j.1365-4632.2011.05007.x
Barron SJ, Del Vecchio MT, Aronoff SC (2014) Intravenous immunoglobulin in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis: a meta-analysis with meta-regression of observational studies. Int J Dermatol. doi:10.1111/ijd.12423
Barron SJ, Del Vecchio MT, Aronoff SC (2015) Intravenous immunoglobulin in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis: a meta-analysis with meta-regression of observational studies. Int J Dermatol 54(1):108–115. doi:10.1111/ijd.12423
Huang YC, Li YC, Chen TJ (2012) The efficacy of intravenous immunoglobulin for the treatment of toxic epidermal necrolysis: a systematic review and meta-analysis. Br J Dermatol 167(2):424–432. doi:10.1111/j.1365-2133.2012.10965.x
Prins C, Kerdel FA, Padilla RS, Hunziker T, Chimenti S, Viard I, Mauri DN, Flynn K, Trent J, Margolis DJ, Saurat JH, French LE (2003) Treatment of toxic epidermal necrolysis with high-dose intravenous immunoglobulins: multicenter retrospective analysis of 48 consecutive cases. Arch Dermatol 139(1):26–32
Valeyrie-Allanore L, Wolkenstein P, Brochard L, Ortonne N, Maitre B, Revuz J, Bagot M, Roujeau JC (2010) Open trial of ciclosporin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol 163(4):847–853. doi:10.1111/j.1365-2133.2010.09863.x
Kirchhof MG, Miliszewski MA, Sikora S, Papp A, Dutz JP (2014) Retrospective review of Stevens-Johnson syndrome/toxic epidermal necrolysis treatment comparing intravenous immunoglobulin with cyclosporine. J Am Acad Dermatol 71(5):941–947. doi:10.1016/j.jaad.2014.07.016
Beylot C, Bioulac P, Doutre MS (1980) Acute generalized exanthematic pustuloses (four cases) (author’s transl). Ann Dermatol Venereol 107(1-2):37–48
Bernard P, Lizeaux-Parneix V, Miossec V et al (1995) HLA et prédisposition génétique dans les pustuloses exanthématiques (PEAG) et les exanthémes maculo-papuleux (EMP). Ann Dermatol Venerol 122:S38–S39
Sidoroff A, Halevy S, Bavinck JN, Vaillant L, Roujeau JC (2001) Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol 28(3):113–119
Roujeau JC, Bioulac-Sage P, Bourseau C, Guillaume JC, Bernard P, Lok C, Plantin P, Claudy A, Delavierre C, Vaillant L et al (1991) Acute generalized exanthematous pustulosis. Analysis of 63 cases. Arch Dermatol 127(9):1333–1338
Leclair MA, Maynard B, St-Pierre C (2009) Acute generalized exanthematous pustulosis with severe organ dysfunction. CMAJ 181(6-7):393–396. doi:10.1503/cmaj.090137
Britschgi M, Pichler WJ (2002) Acute generalized exanthematous pustulosis, a clue to neutrophil-mediated inflammatory processes orchestrated by T cells. Curr Opin Allergy Clin Immunol 2(4):325–331
Stenerson M, Dufendach K, Aksentijevich I, Brady J, Austin J, Reed AM (2011) The first reported case of compound heterozygous IL1RN mutations causing deficiency of the interleukin-1 receptor antagonist. Arthritis Rheum 63(12):4018–4022. doi:10.1002/art.30565
Navarini AA, Valeyrie-Allanore L, Setta-Kaffetzi N, Barker JN, Capon F, Creamer D, Roujeau JC, Sekula P, Simpson MA, Trembath RC, Mockenhaupt M, Smith CH (2013) Rare variations in IL36RN in severe adverse drug reactions manifesting as acute generalized exanthematous pustulosis. J Invest Dermatol 133(7):1904–1907. doi:10.1038/jid.2013.44
Tresch S, Cozzio A, Kamarashev J, Harr T, Schmid-Grendelmeier P, French LE, Feldmeyer L (2012) T cell-mediated acute localized exanthematous pustulosis caused by finasteride. J Allergy Clin Immunol 129(2):589–594. doi:10.1016/j.jaci.2011.07.033
Tetsuo Shiohara YK, Ryo Takahashi, Tadashi Ishida and Yoshiko Mizukawa (2012) Adverse cutaneous drug eruptions. In: LE F (ed) Drug-induced hypersensitivity syndrome (DIHS/DRESS): Recent advances in the diagnosis, pathogenesis and management, vol 1. Karger, Basel, pp 122-135
Shiohara TTR, Kano Y (2007) Drug hypersensitivity. In: Pichler W (ed) Drug-induced hypersensitivity syndrome and viral reactivation, vol 1. Karger, Basel, pp pp 251–266
Cacoub P, Musette P, Descamps V, Meyer O, Speirs C, Finzi L, Roujeau JC (2011) The DRESS syndrome: a literature review. Am J Med 124(7):588–597. doi:10.1016/j.amjmed.2011.01.017
Takahashi R, Kano Y, Yamazaki Y, Kimishima M, Mizukawa Y, Shiohara T (2009) Defective regulatory T cells in patients with severe drug eruptions: timing of the dysfunction is associated with the pathological phenotype and outcome. J Immunol 182(12):8071–8079. doi:10.4049/jimmunol.0804002
Picard D, Janela B, Descamps V, D’Incan M, Courville P, Jacquot S, Rogez S, Mardivirin L, Moins-Teisserenc H, Toubert A, Benichou J, Joly P, Musette P (2010) Drug reaction with eosinophilia and systemic symptoms (DRESS): a multiorgan antiviral T cell response. Sci Transl Med 2:46–46ra62. doi:10.1126/scitranslmed.3001116
Descamps V, Valance A, Edlinger C, Fillet AM, Grossin M, Lebrun-Vignes B, Belaich S, Crickx B (2001) Association of human herpesvirus 6 infection with drug reaction with eosinophilia and systemic symptoms. Arch Dermatol 137(3):301–304
Suzuki Y, Inagi R, Aono T, Yamanishi K, Shiohara T (1998) Human herpesvirus 6 infection as a risk factor for the development of severe drug-induced hypersensitivity syndrome. Arch Dermatol 134(9):1108–1112
Shiohara T, Iijima M, Ikezawa Z, Hashimoto K (2007) The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br J Dermatol 156(5):1083–1084. doi:10.1111/j.1365-2133.2007.07807.x
Kano Y, Hiraharas K, Sakuma K, Shiohara T (2006) Several herpesviruses can reactivate in a severe drug-induced multiorgan reaction in the same sequential order as in graft-versus-host disease. Br J Dermatol 155(2):301–306. doi:10.1111/j.1365-2133.2006.07238.x
Descamps V, Mahe E, Houhou N, Abramowitz L, Rozenberg F, Ranger-Rogez S, Crickx B (2003) Drug-induced hypersensitivity syndrome associated with Epstein-Barr virus infection. Br J Dermatol 148(5):1032–1034
Mardivirin L, Descamps V, Lacroix A, Delebassee S, Ranger-Rogez S (2009) Early effects of drugs responsible for DRESS on HHV-6 replication in vitro. J Clin Virol: Off Publ Pan Am Soc Clin Virol 46(3):300–302. doi:10.1016/j.jcv.2009.08.006
Descamps V, Ranger-Rogez S (2014) DRESS syndrome. Joint Bone Spine: Revue Rhum 81(1):15–21. doi:10.1016/j.jbspin.2013.05.002
Bircher AJ (2011) Uncomplicated drug-induced disseminated exanthemas. In: French L (ed) Adverse cutaneous drug eruptions, vol 1. Karger, Basel, pp 79–96
Funding
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Informed consent
Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
This article is a contribution to the Special Issue on Immunodermatology - Guest Editors: Lars French and Alexander Navarini
Rights and permissions
About this article
Cite this article
Hoetzenecker, W., Nägeli, M., Mehra, E.T. et al. Adverse cutaneous drug eruptions: current understanding. Semin Immunopathol 38, 75–86 (2016). https://doi.org/10.1007/s00281-015-0540-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-015-0540-2