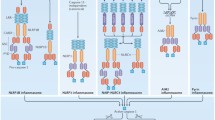
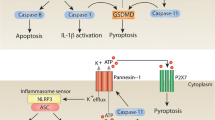
Abstract
Caspase-1 is a unique cysteine protease playing central roles in innate immunity. Pathogens, stress, and damage signals induce activation of caspase-1, typically mediated by proximity-induced autoproteolysis in multimeric protein complexes called the inflammasome. Active caspase-1 induces secretion of pro-inflammatory cytokines and mediates pyroptosis, a programmed pro-inflammatory cell death, thereby initiating an immune response finally leading to pathogen clearance. Excessive activation of caspase-1 is the underlying cause for rare diseases such as periodic fever syndromes, and more common disorders, including atherosclerosis, type 2 diabetes, and gout. Beside these well-known pro-inflammatory functions, active caspase-1 also has anti-inflammatory and protective functions contributing to cell survival, reduced inflammatory cytokine signaling, and improved outcomes in mouse models of burn injury or trauma and shock. Furthermore, naturally occurring procaspase-1 variants with reduced or abrogated enzymatic activity mediate enhanced inflammatory signaling and have been associated to autoinflammatory symptoms. Here, we review functions of caspase-1 focusing on anti-inflammatory signaling pathways and discuss the role of enzymatically inactive caspase-1 as disease-promoting factors in autoinflammatory diseases. Moreover, we illustrate differential requirements for autoproteolysis and enzymatic activity in caspase-1 functions.


Similar content being viewed by others
References
Chowdhury I, Tharakan B, Bhat GK (2008) Caspases — an update. Comp Biochem Physiol B Biochem Mol Biol 151:10–27. doi:10.1016/j.cbpb.2008.05.010
Martinon F, Mayor A, Tschopp J (2009) The inflammasomes: guardians of the body. Annu Rev Immunol 27:229–265. doi:10.1146/annurev.immunol.021908.132715
McIlwain DR, Berger T, Mak TW (2013) Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol 5:a008656. doi:10.1101/cshperspect.a008656
Martinon F, Tschopp J (2004) Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell 117:561–574. doi:10.1016/j.cell.2004.05.004
Yazdi AS, Guarda G, D’Ombrain MC, Drexler SK (2010) Inflammatory caspases in innate immunity and inflammation. J Innate Immun 2:228–237
Kostura MJ, Tocci MJ, Limjuco G et al (1989) Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc Natl Acad Sci U S A 86:5227–5231
Black RA, Kronheim SR, Merriam JE et al (1989) A pre-aspartate-specific protease from human leukocytes that cleaves pro-interleukin-1 beta. J Biol Chem 264:5323–5326
Thornberry NA, Bull HG, Calaycay JR et al (1992) A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 356:768–774. doi:10.1038/356768a0
Cerretti DP, Kozlosky CJ, Mosley B et al (1992) Molecular cloning of the interleukin-1 beta converting enzyme. Science 256:97–100
Walker NP, Talanian RV, Brady KD et al (1994) Crystal structure of the cysteine protease interleukin-1 beta-converting enzyme: a (p20/p10)2 homodimer. Cell 78:343–352
Romanowski MJ, Scheer JM, O’Brien T, McDowell RS (2004) Crystal structures of a ligand-free and malonate-bound human caspase-1: implications for the mechanism of substrate binding. Structure 12:1361–1371. doi:10.1016/j.str.2004.05.010
Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10:417–426
Lamkanfi M, Dixit VM (2012) Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol 28:137–161. doi:10.1146/annurev-cellbio-101011-155745
Faustin B, Lartigue L, Bruey J-M et al (2007) Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell 25:713–724. doi:10.1016/j.molcel.2007.01.032
Boyden ED, Dietrich WF (2006) Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet 38:240–244. doi:10.1038/ng1724
Feldmeyer L, Keller M, Niklaus G et al (2007) The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr Biol 17:1140–1145. doi:10.1016/j.cub.2007.05.074
Broderick L, De Nardo D, Franklin BS et al (2014) The inflammasome and autoinflammatory syndromes. Annu Rev Pathol 10:141125121526004. doi:10.1146/annurev-pathol-012414-040431
Latz E, Xiao TS, Stutz A (2013) Activation and regulation of the inflammasomes. Nat Publ Group 13:397–411. doi:10.1038/nri3452
Newton, K, & Dixit, VM (2012) Signaling in innate immunity and inflammation. Cold Spring Harbor Perspectives in Biology, 4(3), pii: a006049. doi:10.1101/cshperspect.a006049
Bauernfeind FG, Horvath G, Stutz A et al (2009) Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 183:787–791. doi:10.4049/jimmunol.0901363
Fernandes-Alnemri T, Kang S, Anderson C et al (2013) Cutting edge: TLR signaling licenses IRAK1 for rapid activation of the NLRP3 inflammasome. J Immunol 191:3995–3999. doi:10.4049/jimmunol.1301681
Schroder K, Sagulenko V, Zamoshnikova A et al (2012) Acute lipopolysaccharide priming boosts inflammasome activation independently of inflammasome sensor induction. Immunobiology. doi:10.1016/j.imbio.2012.07.020
Py BF, Kim M-S, Vakifahmetoglu-Norberg H, Yuan J (2013) Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell 49:331–338. doi:10.1016/j.molcel.2012.11.009
Juliana C, Fernandes-Alnemri T, Kang S et al (2012) Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem 287:36617–36622. doi:10.1074/jbc.M112.407130
Strowig T, Henao-Mejia J, Elinav E, Flavell R (2012) Inflammasomes in health and disease. Nature 481:278–286. doi:10.1038/nature10759
McDermott MF, Aksentijevich I, Galon J et al (1999) Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell 97:133–144
Almeida de Jesus A, Goldbach-Mansky R (2013) Monogenic autoinflammatory diseases: concept and clinical manifestations. Clin Immunol 147:155–174. doi:10.1016/j.clim.2013.03.016
Hoffman HM, Mueller JL, Broide DH et al (2001) Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet 29:301–305. doi:10.1038/ng756
Aksentijevich I, Nowak M, Mallah M et al (2002) De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum 46:3340–3348. doi:10.1002/art.10688
Feldmann J, Prieur A-M, Quartier P et al (2002) Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet 71:198–203
Masters SL, Simon A, Aksentijevich I, Kastner DL (2009) Horror autoinflammaticus: the Molecular pathophysiology of autoinflammatory disease. Annu Rev Immunol 27:621–668. doi:10.1146/annurev.immunol.25.022106.141627
Kastner DL, Aksentijevich I, Goldbach-Mansky R (2010) Autoinflammatory disease reloaded: a clinical perspective. Cell 140:784–790. doi:10.1016/j.cell.2010.03.002
Keller M, Rüegg A, Werner S, Beer H-D (2008) Active caspase-1 is a regulator of unconventional protein secretion. Cell 132:818–831. doi:10.1016/j.cell.2007.12.040
Bulau A-M, Nold MF, Li S et al (2014) Role of caspase-1 in nuclear translocation of IL-37, release of the cytokine, and IL-37 inhibition of innate immune responses. Proc Natl Acad Sci U S A. doi:10.1073/pnas.1324140111
Gurcel L, Abrami L, Girardin S et al (2006) Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell 126:1135–1145. doi:10.1016/j.cell.2006.07.033
Jabir MS, Ritchie ND, Li D et al (2014) Caspase-1 cleavage of the TLR adaptor TRIF inhibits autophagy and β-interferon production during Pseudomonas aeruginosa infection. Cell Host Microbe 15:214–227. doi:10.1016/j.chom.2014.01.010
Madouri F, Guillou N, Fauconnier L et al (2015) Caspase-1 activation by NLRP3 inflammasome dampens IL-33-dependent house dust mite-induced allergic lung inflammation. J Mol Cell Biol. doi:10.1093/jmcb/mjv012
Osuka A, Hanschen M, Stoecklein V, Lederer JA (2012) A protective role for inflammasome activation following injury. Shock 37:47–55. doi:10.1097/SHK.0b013e318234f7ff
Menzel CL, Sun Q, Loughran PA et al (2011) Caspase-1 is hepatoprotective during trauma and hemorrhagic shock by reducing liver injury and inflammation. Mol Med 17:1031–1038. doi:10.2119/molmed.2011.00015
Luksch H, Romanowski MJ, Chara O et al (2013) Naturally occurring genetic variants of human caspase-1 differ considerably in structure and the ability to activate interleukin-1β. Hum Mutat 34:122–131. doi:10.1002/humu.22169
Heymann MC, Winkler S, Luksch H et al (2014) Human procaspase-1 variants with decreased enzymatic activity are associated with febrile episodes and may contribute to inflammation via RIP2 and NF-κB signaling. J Immunol 192:4379–4385. doi:10.4049/jimmunol.1203524
Li P (1995) Mice deficient in IL-1b-converting enzyme are defective in production of mature IL-1b and resistant to endotoxic shock. Cell 80:401–411. doi:10.1016/0092-8674(95)90490-5
Kuida K, Lippke JA, Ku G et al (1995) Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science 267:2000–2003
Zychlinsky A, Prevost MC, Sansonetti PJ (1992) Shigella flexneri induces apoptosis in infected macrophages. Nature 358:167–169. doi:10.1038/358167a0
Miao EA, Rajan JV, Aderem A (2011) Caspase-1-induced pyroptotic cell death. Immunol Rev 243:206–214. doi:10.1111/j.1600-065X.2011.01044.x
Bergsbaken T, Fink SL, Cookson BT (2009) Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7:99–109. doi:10.1038/nrmicro2070
Dinarello CA (2009) Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27:519–550. doi:10.1146/annurev.immunol.021908.132612
Miggin SM, Palsson-McDermott E, Dunne A et al (2007) NF-κB activation by the Toll-IL-1 receptor domain protein MyD88 adapter-like is regulated by caspase-1. Proc Natl Acad Sci U S A 104:3372–3377. doi:10.1073/pnas.0608100104
Erener S, Pétrilli V, Kassner I et al (2012) Inflammasome-activated caspase 7 cleaves PARP1 to enhance the expression of a subset of NF-κB target genes. Mol Cell. doi:10.1016/j.molcel.2012.02.016
Lamkanfi M, Kalai M, Saelens X et al (2004) Caspase-1 activates nuclear factor of the kappa-enhancer in B cells independently of its enzymatic activity. J Biol Chem 279:24785–24793. doi:10.1074/jbc.M400985200
Kersse K, Lamkanfi M, Bertrand MJM et al (2011) Interaction patches of procaspase-1 caspase recruitment domains (CARDs) Are differently involved in procaspase-1 activation and receptor-interacting protein 2 (RIP2)-dependent nuclear factor B signaling. J Biol Chem 286:35874–35882. doi:10.1074/jbc.M111.242321
Sarkar A, Duncan M, Hart J et al (2006) ASC directs NF-kappaB activation by regulating receptor interacting protein-2 (RIP2) caspase-1 interactions. J Immunol 176:4979–4986
Broadley KN, Aquino AM, Woodward SC et al (1989) Monospecific antibodies implicate basic fibroblast growth factor in normal wound repair. Lab Investig 61:571–575
Kumar S, Hanning CR, Brigham-Burke MR et al (2002) Interleukin-1F7B (IL-1H4/IL-1F7) is processed by caspase-1 and mature IL-1F7B binds to the IL-18 receptor but does not induce IFN-gamma production. Cytokine 18:61–71. doi:10.1006/cyto.2002.0873
Sharma S, Kulk N, Nold MF et al (2008) The IL-1 family member 7b translocates to the nucleus and down-regulates proinflammatory cytokines. J Immunol 180:5477–5482
Nold MF, Nold-Petry CA, Zepp JA et al (2010) IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol 11:1014–1022. doi:10.1038/ni.1944
Moretti S, Bozza S, Oikonomou V et al (2014) IL-37 inhibits inflammasome activation and disease severity in murine aspergillosis. PLoS Pathog 10:e1004462. doi:10.1371/journal.ppat.1004462
Mao K, Chen S, Chen M et al (2013) Nitric oxide suppresses NLRP3 inflammasome activation and protects against LPSinduced septic shock. Cell Res 23:201–212. doi:10.1038/cr.2013.6
Koizumi Y, Toma C, Higa N et al (2011) Inflammasome activation via intracellular NLRs triggered by bacterial infection. Cell Microbiol 14:149–154. doi:10.1111/j.1462-5822.2011.01707.x
Goldstein JL, DeBose-Boyd RA, Brown MS (2006) Protein sensors for membrane sterols. Cell 124:35–46. doi:10.1016/j.cell.2005.12.022
Aries A, Whitcomb J, Shao W et al (2014) Caspase-1 cleavage of transcription factor GATA4 and regulation of cardiac cell fate. Cell Death Dis 5:e1566. doi:10.1038/cddis.2014.524
Deretic V, Levine B (2009) Autophagy, immunity, and microbial adaptations. Cell Host Microbe 5:527–549. doi:10.1016/j.chom.2009.05.016
Baehrecke EH (2005) Autophagy: dual roles in life and death? Nat Rev Mol Cell Biol 6:505. doi:10.1038/nrm1666
Rebsamen M, Meylan E, Curran J, Tschopp J (2008) The antiviral adaptor proteins Cardif and Trif are processed and inactivated by caspases. Cell Death Differ 15:1804–1811. doi:10.1038/cdd.2008.119
Kayagaki N, Warming S, Lamkanfi M et al (2011) Non-canonical inflammasome activation targets caspase-11. Nature 479:117–121. doi:10.1038/nature10558
Rathinam VAK, Vanaja SK, Waggoner L et al (2012) TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 150:606–619. doi:10.1016/j.cell.2012.07.007
Gurung P, Malireddi RKS, Anand PK et al (2012) Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-β (TRIF)-mediated caspase-11 protease production integrates Toll-like receptor 4 (TLR4) protein- and Nlrp3 inflammasome-mediated host defense against enteropathogens. J Biol Chem 287:34474–34483. doi:10.1074/jbc.M112.401406
Henry T, Brotcke A, Weiss DS et al (2007) Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J Exp Med 204:987–994. doi:10.1084/jem.20062665
Makrinioti H, Toussaint M, Jackson DJ et al (2014) Role of interleukin 33 in respiratory allergy and asthma. Lancet Respir Med 2:226–237. doi:10.1016/S2213-2600(13)70261-3
Pei C, Barbour M, Fairlie-Clarke KJ et al (2013) Emerging role of interleukin-33 in autoimmune diseases. Immunology 141:9–17. doi:10.1111/imm.12174
Kakkar R, Lee RT (2008) The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov 7:827–840. doi:10.1038/nrd2660
Cayrol C, Girard J-P (2009) The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci U S A 106:9021–9026. doi:10.1073/pnas.0812690106
Kool M, Willart MAM, van Nimwegen M et al (2011) An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity 34:527–540. doi:10.1016/j.immuni.2011.03.015
Hoffmann A, Baltimore D (2006) Circuitry of nuclear factor kappaB signaling. Immunol Rev 210:171–186. doi:10.1111/j.0105-2896.2006.00375.x
Ogura Y, Inohara N, Benito A et al (2001) Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem 276:4812–4818. doi:10.1074/jbc.M008072200
Mariathasan S, Newton K, Monack DM et al (2004) Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430:213–218. doi:10.1038/nature02664
Groß O, Yazdi AS, Thomas CJ et al (2012) Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity 36:388–400. doi:10.1016/j.immuni.2012.01.018
Broz P, von Moltke J, Jones JW et al (2010) Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 8:471–483. doi:10.1016/j.chom.2010.11.007
Guey B, Bodnar M, Manié SN et al (2014) Caspase-1 autoproteolysis is differentially required for NLRP1b and NLRP3 inflammasome function. Proc Natl Acad Sci U S A 111:17254–17259. doi:10.1073/pnas.1415756111
Van Opdenbosch N, Gurung P, Vande Walle L et al (2014) Activation of the NLRP1b inflammasome independently of ASC-mediated caspase-1 autoproteolysis and speck formation. Nat Commun 5:3209. doi:10.1038/ncomms4209
Acknowledgments
We thank Christian Hedrich and Jochen Roesler for fruitful discussions and critical reading of the manuscript.
Conflicts of interest
The authors declare that they have no competing interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is a contribution to the Special Issue on The Inflammasome and Autoinflammatory Diseases - Guest Editors: Seth L. Masters, Tilmann Kallinich and Seza Ozen
Rights and permissions
About this article
Cite this article
Winkler, S., Rösen-Wolff, A. Caspase-1: an integral regulator of innate immunity. Semin Immunopathol 37, 419–427 (2015). https://doi.org/10.1007/s00281-015-0494-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-015-0494-4