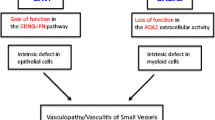
Abstract
Translating pathogenic insights gained from monogenic defects that cause autoinflammatory diseases into novel therapies has dramatically improved the lives of patients with these syndromes. The last 15 years have focused on the central role of IL-1 in driving autoinflammatory phenotypes and on therapies blocking IL-1 signaling. Recent discoveries from patients unresponsive to IL-1 blockade have highlighted other key inflammatory mediators and pathways. New genetic discoveries have confirmed unifying mechanisms of autoinflammation, including dysregulation of danger sensing, cell stress, and immune-receptor signaling. Recent gene discovery in novel diseases has demonstrated new concepts. First, several complex clinical syndromes, caused by mutations leading to chronic type I interferon (IFN) production present with organ manifestations different from IL-1 mediated diseases including cerebral calcifications, myositis, and interstitial lung disease and the frequent occurrence of autoantibodies. These disorders introduce type I IFN’s as inflammatory mediators that cause autoinflammatory phenotypes. Second, conditions associated with high IL-18 production may provide a direct link between autoinflammation and macrophage activation syndrome. Third, dysregulation of inflammatory and cell differentiation pathways in nonhematopoietic cells, such as aberrant calcium signaling and impaired endothelial or keratinocyte development, provide an understanding of organ specificity in autoinflammatory disorders. Many of these discoveries highlight the intricate interconnections between autoinflammation, autoimmunity, immunodeficiency, and lymphoproliferation and suggest ways in which we may better diagnose and treat autoinflammatory diseases.


Similar content being viewed by others
References
McDermott MF, Aksentijevich I, Galon J et al (1999) Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell 97:133–144
Goldbach-Mansky R, Kastner DL (2009) Autoinflammation: the prominent role of IL-1 in monogenic autoinflammatory diseases and implications for common illnesses. J Allergy Clin Immunol 124:1141–1149, quiz 50-1
Gonzalez-Navajas JM, Lee J, David M, Raz E (2012) Immunomodulatory functions of type I interferons. Nat Rev Immunol 12:125–135
Schoggins JW, Wilson SJ, Panis M et al (2011) A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472:481–485
Bennett L, Palucka AK, Arce E et al (2003) Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med 197:711–723
Agarwal AK, Xing C, DeMartino GN et al (2010) PSMB8 encoding the beta5i proteasome subunit is mutated in joint contractures, muscle atrophy, microcytic anemia, and panniculitis-induced lipodystrophy syndrome. Am J Hum Genet 87:866–872
Arima K, Kinoshita A, Mishima H et al (2011) Proteasome assembly defect due to a proteasome subunit beta type 8 (PSMB8) mutation causes the autoinflammatory disorder, Nakajo-Nishimura syndrome. Proc Natl Acad Sci U S A 108:14914–14919
Kitamura A, Maekawa Y, Uehara H et al (2011) A mutation in the immunoproteasome subunit PSMB8 causes autoinflammation and lipodystrophy in humans. J Clin Invest 121:4150–4160
Liu Y, Ramot Y, Torrelo A et al (2012) Mutations in proteasome subunit beta type 8 cause chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature with evidence of genetic and phenotypic heterogeneity. Arthritis Rheum 64:895–907
Lu W, Zhang Y, McDonald DO et al (2014) Dual proteolytic pathways govern glycolysis and immune competence. Cell 159:1578–1590
Liu Y, Jesus AA, Marrero B et al (2014) Activated STING in a vascular and pulmonary syndrome. N Engl J Med 371:507–518
Jeremiah N, Neven B, Gentili M et al (2014) Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J Clin Invest 124:5516–5520
Aicardi J, Goutieres F (1984) A progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann Neurol 15:49–54
Crow YJ, Chase DS, Lowenstein Schmidt J et al (2015) Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am J Med Genet A 167A:296–312
Rice GI, del Toro Duany Y, Jenkinson EM et al (2014) Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nat Genet 46:503–509
Bogunovic D, Byun M, Durfee LA et al (2012) Mycobacterial disease and impaired IFN-gamma immunity in humans with inherited ISG15 deficiency. Science 337:1684–1688
Zhang X, Bogunovic D, Payelle-Brogard B et al (2015) Human intracellular ISG15 prevents interferon-alpha/beta over-amplification and auto-inflammation. Nature 517:89–93
Briggs TA, Rice GI, Daly S et al (2011) Tartrate-resistant acid phosphatase deficiency causes a bone dysplasia with autoimmunity and a type I interferon expression signature. Nat Genet 43:127–131
Lausch E, Janecke A, Bros M et al (2011) Genetic deficiency of tartrate-resistant acid phosphatase associated with skeletal dysplasia, cerebral calcifications and autoimmunity. Nat Genet 43:132–137
Stepp SE, Dufourcq-Lagelouse R, Le Deist F et al (1999) Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science 286:1957–1959
Pachlopnik Schmid J, Cote M, Menager MM et al (2010) Inherited defects in lymphocyte cytotoxic activity. Immunol Rev 235:10–23
Villanueva J, Lee S, Giannini EH et al (2005) Natural killer cell dysfunction is a distinguishing feature of systemic onset juvenile rheumatoid arthritis and macrophage activation syndrome. Arthritis Res Ther 7:R30–R37
Kaufman KM, Linghu B, Szustakowski JD et al (2014) Whole-exome sequencing reveals overlap between macrophage activation syndrome in systemic juvenile idiopathic arthritis and familial hemophagocytic lymphohistiocytosis. Arthritis Rheumatol 66:3486–3495
Rose NR, Hamilton RG, Detrick B (2002) Manual of clinical laboratory immunology, 6th edn. ASM Press, Washington D.C
Romberg N, Al Moussawi K, Nelson-Williams C, et al. (2014) Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat Genet 46:1135–1139
Canna SW, de Jesus AA, Gouni S, et al. (2014) An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet 46:1140–1146
Ichida H, Kawaguchi Y, Sugiura T, et al. (2013) Clinical manifestations of adult-onset still's disease presenting with erosive arthritis: association with low levels of ferritin and IL-18. Arthritis care & Res 66:642--646
Shimizu M, Nakagishi Y, Yachie A (2013) Distinct subsets of patients with systemic juvenile idiopathic arthritis based on their cytokine profiles. Cytokine 61:345–348
Kitamura A, Sasaki Y, Abe T, Kano H, Yasutomo K (2014) An inherited mutation in NLRC4 causes autoinflammation in human and mice. J Exp Med 211:2385–2396
Rigaud S, Fondaneche MC, Lambert N et al (2006) XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature 444:110–114
Marsh RA, Madden L, Kitchen BJ et al (2010) XIAP deficiency: a unique primary immunodeficiency best classified as X-linked familial hemophagocytic lymphohistiocytosis and not as X-linked lymphoproliferative disease. Blood 116:1079–1082
Aguilar C, Lenoir C, Lambert N, et al. (2014) Characterization of Crohn disease in X-linked inhibitor of apoptosis-deficient male patients and female symptomatic carriers. J allergy and Clin Immunol 134:1131--1141
Speckmann C, Lehmberg K, Albert MH et al (2013) X-linked inhibitor of apoptosis (XIAP) deficiency: the spectrum of presenting manifestations beyond hemophagocytic lymphohistiocytosis. Clin Immunol 149:133–141
Wada T, Kanegane H, Ohta K et al (2014) Sustained elevation of serum interleukin-18 and its association with hemophagocytic lymphohistiocytosis in XIAP deficiency. Cytokine 65:74–78
Damgaard RB, Gyrd-Hansen M (2011) Inhibitor of apoptosis (IAP) proteins in regulation of inflammation and innate immunity. Discovery Med 11:221–231
Damgaard RB, Nachbur U, Yabal M et al (2012) The ubiquitin ligase XIAP recruits LUBAC for NOD2 signaling in inflammation and innate immunity. Mol Cell 46:746–758
de Jesus AA, Canna SW, Liu Y, Goldbach-Mansky R. (2015) Molecular mechanisms in genetically defined autoinflammatory diseases: disorders of amplified danger signaling. Annu Rev Immunol 33:823--874
Yabal M, Muller N, Adler H et al (2014) XIAP restricts TNF- and RIP3-dependent cell death and inflammasome activation. Cell Reports 7:1796–1808
Jordan CT, Cao L, Roberson ED et al (2012) Rare and common variants in CARD14, encoding an epidermal regulator of NF-kappaB, in psoriasis. Am J Hum Genet 90:796–808
Jordan CT, Cao L, Roberson ED et al (2012) PSORS2 is due to mutations in CARD14. Am J Hum Genet 90:784–795
Fuchs-Telem D, Sarig O, van Steensel MA et al (2012) Familial pityriasis rubra pilaris is caused by mutations in CARD14. Am J Hum Genet 91:163–170
Eytan O, Sarig O, Sprecher E, van Steensel MA (2014) Clinical response to ustekinumab in familial pityriasis rubra pilaris caused by a novel mutation in CARD14. British J Dermatol 171:420–422
Zhou Q, Yang D, Ombrello AK et al (2014) Early-onset stroke and vasculopathy associated with mutations in ADA2. N Engl J Med 370:911–920
Navon Elkan P, Pierce SB, Segel R et al (2014) Mutant adenosine deaminase 2 in a polyarteritis nodosa vasculopathy. N Engl J Med 370:921–931
Chakraborty PK, Schmitz-Abe K, Kennedy EK et al (2014) Mutations in TRNT1 cause congenital sideroblastic anemia with immunodeficiency, fevers, and developmental delay (SIFD). Blood 124:2867–2871
Wiseman DH, May A, Jolles S et al (2013) A novel syndrome of congenital sideroblastic anemia, B-cell immunodeficiency, periodic fevers, and developmental delay (SIFD). Blood 122:112–123
Sasarman F, Thiffault I, Weraarpachai W, et al. (2015) The 3' addition of CCA to mitochondrial tRNASer(AGY) is specifically impaired in patients with mutations in the tRNA nucleotidyl transferase TRNT1. Human Mol genet 24:2841--2847
Ombrello MJ, Remmers EF, Sun G et al (2012) Cold urticaria, immunodeficiency, and autoimmunity related to PLCG2 deletions. N Engl J Med 366:330–338
Chae JJ, Park YH, Park C, et al. (2014) Connecting two pathways through Ca signaling: NLRP3 inflammasome activation induced by a hypermorphic PLCG2 mutation. Arthritis & Rheumatol 67:563--567
Livingston JH, Lin JP, Dale RC et al (2014) A type I interferon signature identifies bilateral striatal necrosis due to mutations in ADAR1. J Med Genet 51:76–82
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases at the NIH
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This article is a contribution to the Special Issue on The Inflammasome and Autoinflammatory Diseases - Guest Editors: Seth L. Masters, Tilmann Kallinich, and Seza Ozen
Rights and permissions
About this article
Cite this article
Canna, S.W., Goldbach-Mansky, R. New monogenic autoinflammatory diseases—a clinical overview. Semin Immunopathol 37, 387–394 (2015). https://doi.org/10.1007/s00281-015-0493-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-015-0493-5