Abstract
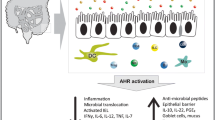
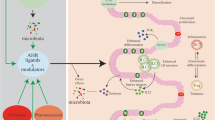
The aryl hydrocarbon receptor (AhR), a transcription factor activated by a large variety of natural and synthetic ligands, has recently become the object of great interest among researchers since it represents an important link between environment and immune-mediated pathologies. In this context, evidence has been accumulated to show that AhR is necessary for the maintenance/expansion of intraepithelial lymphocytes and interleukin-22-producing innate lymphoid cells in the gut and that defects in AhR-delivered signals may contribute to amplify gut tissue destructive immune-inflammatory reactions. We here review the available data supporting the role of AhR in the control of immune homeostasis in the gut and discuss whether and how AhR activators can help dampen inflammatory processes.

Similar content being viewed by others
References
Kaser A, Zeissig S, Blumberg RS (2010) Inflammatory bowel disease. Annu Rev Immunol 28:573–621. doi:10.1146/annurev-immunol-030409-101225
Strober W, Fuss IJ (2011) Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 140(6):1756–1767. doi:10.1053/j.gastro.2011.02.016
MacDonald TT, Monteleone I, Fantini MC, Monteleone G (2011) Regulation of homeostasis and inflammation in the intestine. Gastroenterology 140(6):1768–1775. doi:10.1053/j.gastro.2011.02.047
Cosnes J, Gower-Rousseau C, Seksik P, Cortot A (2011) Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 140(6):1785–1794. doi:10.1053/j.gastro.2011.01.055
Cosnes J (2004) Tobacco and IBD: relevance in the understanding of disease mechanisms and clinical practice. Best Pract Res Clin Gastroenterol 18(3):481–496. doi:10.1016/j.bpg.2003.12.003
Hou JK, Abraham B, El-Serag H (2011) Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol 106(4):563–573. doi:10.1038/ajg.2011.44
Godet PG, May GR, Sutherland LR (1995) Meta-analysis of the role of oral contraceptive agents in inflammatory bowel disease. Gut 37(5):668–673
Gu YZ, Hogenesch JB, Bradfield CA (2000) The PAS superfamily: sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol 40:519–561. doi:10.1146/annurev.pharmtox.40.1.519
Denison MS, Nagy SR (2003) Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol 43:309–334. doi:10.1146/annurev.pharmtox.43.100901.135828
Rannug U, Rannug A, Sjoberg U, Li H, Westerholm R, Bergman J (1995) Structure elucidation of two tryptophan-derived, high affinity Ah receptor ligands. Chem Biol 2(12):841–845
Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, Wilhelm C, Veldhoen M (2011) Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 147(3):629–640. doi:10.1016/j.cell.2011.09.025
Cheroutre H, Lambolez F, Mucida D (2011) The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol 11(7):445–456. doi:10.1038/nri3007
Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, Mantovani A, Kopan R, Bradfield CA, Newberry RD, Colonna M (2011) AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol 13(2):144–151. doi:10.1038/ni.2187
Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, Diefenbach A (2011) Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 334(6062):1561–1565. doi:10.1126/science.1214914
Spits H, Di Santo JP (2011) The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol 12(1):21–27. doi:10.1038/ni.1962
Alam MS, Maekawa Y, Kitamura A, Tanigaki K, Yoshimoto T, Kishihara K, Yasutomo K (2010) Notch signaling drives IL-22 secretion in CD4+ T cells by stimulating the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A. doi:10.1073/pnas.0911755107
Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, Soumelis V (2008) A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol 9(6):650–657
Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR (2006) The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126(6):1121–1133. doi:10.1016/j.cell.2006.07.035
Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y (2003) Increased expression of interleukin 17 in inflammatory bowel disease. Gut 52(1):65–70
Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S (2007) Phenotypic and functional features of human Th17 cells. J Exp Med 204(8):1849–1861
Seiderer J, Elben I, Diegelmann J, Glas J, Stallhofer J, Tillack C, Pfennig S, Jurgens M, Schmechel S, Konrad A, Goke B, Ochsenkuhn T, Muller-Myhsok B, Lohse P, Brand S (2008) Role of the novel Th17 cytokine IL-17F in inflammatory bowel disease (IBD): upregulated colonic IL-17F expression in active Crohn’s disease and analysis of the IL17F p.His161Arg polymorphism in IBD. Inflamm Bowel Dis 14(4):437–445. doi:10.1002/ibd.20339
Monteleone G, Biancone L, Marasco R, Morrone G, Marasco O, Luzza F, Pallone F (1997) Interleukin 12 is expressed and actively released by Crohn’s disease intestinal lamina propria mononuclear cells. Gastroenterology 112(4):1169–1178
Schmidt C, Giese T, Ludwig B, Mueller-Molaian I, Marth T, Zeuzem S, Meuer SC, Stallmach A (2005) Expression of interleukin-12-related cytokine transcripts in inflammatory bowel disease: elevated interleukin-23p19 and interleukin-27p28 in Crohn’s disease but not in ulcerative colitis. Inflamm Bowel Dis 11(1):16–23
Caruso R, Sarra M, Stolfi C, Rizzo A, Fina D, Fantini MC, Pallone F, MacDonald TT, Monteleone G (2009) Interleukin-25 inhibits interleukin-12 production and Th1 cell-driven inflammation in the gut. Gastroenterology 136(7):2270–2279. doi:10.1053/j.gastro.2009.02.049
Iliev ID, Spadoni I, Mileti E, Matteoli G, Sonzogni A, Sampietro GM, Foschi D, Caprioli F, Viale G, Rescigno M (2009) Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut 58(11):1481–1489. doi:10.1136/gut.2008.175166
Rodriguez-Sosa M, Elizondo G, Lopez-Duran RM, Rivera I, Gonzalez FJ, Vega L (2005) Over-production of IFN-gamma and IL-12 in AhR-null mice. FEBS Lett 579(28):6403–6410. doi:10.1016/j.febslet.2005.10.023
Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B (2008) The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453(7191):106–109. doi:10.1038/nature06881
Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL (2008) Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453(7191):65–71. doi:10.1038/nature06880
Quintana FJ, Jin H, Burns EJ, Nadeau M, Yeste A, Kumar D, Rangachari M, Zhu C, Xiao S, Seavitt J, Georgopoulos K, Kuchroo VK (2012) Aiolos promotes TH17 differentiation by directly silencing Il2 expression. Nat Immunol 13(8):770–777. doi:10.1038/ni.2363
Monteleone I, Rizzo A, Sarra M, Sica G, Sileri P, Biancone L, Macdonald TT, Pallone F, Monteleone G (2011) Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology 141(1):237–248. doi:10.1053/j.gastro.2011.04.007, e231
Arsenescu R, Arsenescu V, Zhong J, Nasser M, Melinte R, Dingle RW, Swanson H, de Villiers WJ (2011) Role of the xenobiotic receptor in inflammatory bowel disease. Inflamm Bowel Dis 17(5):1149–1162. doi:10.1002/ibd.21463
Strober W, Fuss IJ, Blumberg RS (2002) The immunology of mucosal models of inflammation. Annu Rev Immunol 20:495–549
Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A (2008) IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest 118(2):534–544. doi:10.1172/JCI33194
Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA (2008) Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 29(6):947–957. doi:10.1016/j.immuni.2008.11.003
Huang Z, Jiang Y, Yang Y, Shao J, Sun X, Chen J, Dong L, Zhang J (2012) 3,3′-Diindolylmethane alleviates oxazolone-induced colitis through Th2/Th17 suppression and Treg induction. Mol Immunol 53(4):335–344. doi:10.1016/j.molimm.2012.09.007
Furumatsu K, Nishiumi S, Kawano Y, Ooi M, Yoshie T, Shiomi Y, Kutsumi H, Ashida H, Fujii-Kuriyama Y, Azuma T, Yoshida M (2011) A role of the aryl hydrocarbon receptor in attenuation of colitis. Dig Dis Sci 56(9):2532–2544. doi:10.1007/s10620-011-1643-9
Takamura T, Harama D, Fukumoto S, Nakamura Y, Shimokawa N, Ishimaru K, Ikegami S, Makino S, Kitamura M, Nakao A (2011) Lactobacillus bulgaricus OLL1181 activates the aryl hydrocarbon receptor pathway and inhibits colitis. Immunol Cell Biol 89(7):817–822. doi:10.1038/icb.2010.165
Perdew GH, Babbs CF (1991) Production of Ah receptor ligands in rat fecal suspensions containing tryptophan or indole-3-carbinol. Nutr Cancer 16(3–4):209–218. doi:10.1080/01635589109514159
Gandhi R, Kumar D, Burns EJ, Nadeau M, Dake B, Laroni A, Kozoriz D, Weiner HL, Quintana FJ (2010) Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol 11(9):846–853. doi:10.1038/ni.1915
Singh NP, Singh UP, Singh B, Price RL, Nagarkatti M, Nagarkatti PS (2011) Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PLoS One 6(8):e23522. doi:10.1371/journal.pone.0023522
Benson JM, Shepherd DM (2011) Aryl hydrocarbon receptor activation by TCDD reduces inflammation associated with Crohn’s disease. Toxicol Sci 120(1):68–78. doi:10.1093/toxsci/kfq360
Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, Burns EJ, Sherr DH, Weiner HL, Kuchroo VK (2010) The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol 11(9):854–861. doi:10.1038/ni.1912
Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, Hu B, Hedl M, Zhang W, O’Connor W Jr, Murphy AJ, Valenzuela DM, Yancopoulos GD, Booth CJ, Cho JH, Ouyang W, Abraham C, Flavell RA (2012) IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 491(7423):259–263. doi:10.1038/nature11535
Acknowledgments
The authors received support for their work on AhR in IBD from Giuliani Spa (Milan, Italy) and “Fondazione Umberto Di Mario” (Rome, Italy).
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is a contribution to the special issue on Roles of Aryl Hydrocarbon Receptor in Controlling Immunity - Guest Editors: C. Pot, V. Kuchroo and F. Quintaña
Rights and permissions
About this article
Cite this article
Monteleone, I., Pallone, F. & Monteleone, G. Aryl hydrocarbon receptor and colitis. Semin Immunopathol 35, 671–675 (2013). https://doi.org/10.1007/s00281-013-0396-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-013-0396-2