Abstract
In the pathogenesis of sepsis, inflammation and coagulation play a pivotal role. Increasing evidence points to an extensive cross-talk between these two systems, whereby inflammation not only leads to activation of coagulation but coagulation also considerably affects inflammatory activity. The intricate relationship between inflammation and coagulation may not only be relevant for vascular atherothrombotic disease in general but has in certain clinical settings considerable consequences, for example in the pathogenesis of microvascular failure and subsequent multiple organ failure, as a result of severe infection and the associated systemic inflammatory response. Molecular pathways that contribute to inflammation-induced activation of coagulation have been precisely identified. Pro-inflammatory cytokines and other mediators are capable of activating the coagulation system and downregulating important physiological anticoagulant pathways. Activation of the coagulation system and ensuing thrombin generation is dependent on an interleukin-6-induced expression of tissue factor on activated mononuclear cells and endothelial cells and is insufficiently counteracted by physiological anticoagulant mechanisms and endogenous fibrinolysis. Interestingly, apart from the overall systemic responses, a differential local response in various vascular beds related to specific organs may occur.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most critically ill patients have an activated coagulation system. This activation of coagulation is measurable with highly sensitive assays for molecular markers of activated coagulation proteases, their activation peptides, or protease–protease inhibitor complexes [1]. In many patients, this activation may go undetected, although in the majority of them some abnormality in routine coagulation tests such as a drop in platelet count or a minor prolongation of global coagulation tests may occur. Most clinicians do not regard these abnormalities very relevant. In more severe forms of coagulation activation, however, it is now clear that the ensuing formation of intravascular fibrin may contribute to the pathogenesis of multiple organ failure, in particular in patients with a systemic inflammatory response, for example due to severe infection or trauma [2]. Indeed, in the majority of patients with disseminated intravascular coagulation, fibrin thrombi can be found in many organs (Table 1) [3, 4].
The pathogenesis of the systemic activation of coagulation and microvascular fibrin formation has become more clear in recent years [5]. The trigger for the activation of the coagulation system is mediated by several pro-inflammatory cytokines, expressed and released by mononuclear cells and endothelial cells. Thrombin generation proceeds via the (extrinsic) tissue factor/factor VIIa route and simultaneously occurring depression of inhibitory mechanisms, such as antithrombin III and the protein C and S system. Also, impaired fibrin degradation, due to high circulating levels of PAI-1, contributes to enhanced intravascular fibrin deposition.
Clinical importance of the interaction between inflammation and coagulation
There is evidence that activation of coagulation in concert with inflammatory activation can result in microvascular thrombosis and thereby contribute to multiple organ failure in patients with severe sepsis [6]. Firstly, there are several reports of post-mortem findings in septic patients with coagulation abnormalities and DIC [7, 8]. These autopsy findings include diffuse bleeding at various sites, hemorraghic necrosis of tissue, microthrombi in small blood vessels, and thrombi in mid-size and larger arteries and veins. The demonstration of ischemia and necrosis was associated with fibrin deposition in small and mid-size vessels of various organs [9]. Importantly, the presence of these intravascular thrombi appears to be clearly and specifically related to the development of organ dysfunction. Secondly, experimental animal studies of DIC show fibrin deposition in various organs. Experimental bacteremia or endotoxemia causes intra- and extravascular fibrin deposition in kidneys, lungs, liver, brain, and various other organs. Amelioration of the hemostatic defect by various interventions in these experimental models appears to improve organ failure and, in some but not all cases, mortality [10–13]. Interestingly, some studies indicate that amelioration of the systemic coagulation activation will have a profound beneficial effect on resolution of local fibrin deposition and improvement of organ failure [14, 15]. Lastly, clinical studies support the notion of coagulation as an important determinant of clinical outcome. DIC has shown to be an independent predictor of organ failure and mortality [2, 16]. In a consecutive series of patients with severe sepsis, the mortality of patients with DIC was 43%, as compared with 27% in those without DIC. In this study, mortality was also directly related to the severity of the coagulopathy in septic patients [17].
Apart from microvascular thrombosis and organ dysfunction, coagulation abnormalities may also have other harmful consequences. For example, thrombocytopenia in patients with sepsis confers an increased risk of bleeding [18]. Indeed, in particular critically ill patients with a platelet count of <50 × 109/l have a 4- to 5-fold higher risk for bleeding as compared to patients with a higher platelet count [19, 20]. The risk of intracerebral bleeding in patients in the intensive care unit (ICU) is relatively low (0.3–0.5%), but in 88% of patients with this complication the platelet count is less than 100 × 109/l [21]. Regardless of the cause, thrombocytopenia is an independent predictor of ICU mortality in multivariate analyses with a relative risk of 1.9 to 4.2 in various studies [19, 22, 23]. In particular, a sustained thrombocytopenia during more than 4 days after ICU admission or a drop in platelet count of >50% during ICU stay is associated with a 4- to 6-fold increase in mortality [19, 24]. The platelet count was shown to be a stronger predictor for ICU mortality than composite scoring systems, such as the Acute Physiology and Chronic Evaluation (APACHE) II score or the Multiple Organ Dysfunction Score (MODS). Also, low levels of coagulation factors in patients with sepsis, as reflected by prolonged global coagulation times, may be a risk factor for bleeding and mortality. A PT or aPTT ratio >1.5 in critically ill patients was found to predict excessive bleeding and increased mortality [25, 26].
Initiation and propagation of inflammation-induced activation of coagulation
Tissue factor plays a central role in the initiation of inflammation-induced coagulation [27]. Blocking tissue factor activity completely inhibits inflammation-induced thrombin generation in models of experimental endotoxemia or bacteremia [12, 28]. The vast majority of cells constitutively expressing tissue factor are found in tissues not in direct contact with blood, such as the adventitial layer of larger blood vessels. However, tissue factor comes into contact with blood when the integrity of the vessel wall is disrupted or when endothelial cells and/or circulating blood cells start expressing tissue factor. The in vivo expression of tissue factor seems mostly dependent on IL-6, as demonstrated in studies showing that inhibition of IL-6 completely abrogates tissue factor-dependent thrombin generation in experimental endotoxemia, whereas specific inhibition of other pro-inflammatory cytokines had less or no effect [29, 30]. Inflammatory cells in atherosclerotic plaques produce abundant tissue factor and upon plaque rupture there is extensive tissue factor exposure to blood [31]. In severe sepsis, mononuclear cells, stimulated by pro-inflammatory cytokines, express tissue factor, which leads to systemic activation of coagulation [32]. Even in experimental low-dose endotoxemia in healthy subjects, a 125-fold increase in tissue factor mRNA levels in blood monocytes can be detected [33]. A potential alternative source of tissue factor may be endothelial cells, polymorphonuclear cells, and other cell types. It is hypothesized that tissue factor from these sources is shuttled between cells through microparticles derived from activated mononuclear cells [34]. It is, however, unlikely that these cells actually synthesize tissue factor in substantial quantities [32, 35].
Upon exposure to blood, tissue factor binds to factor VIIa. The complex of tissue factor–factor VIIa catalyzes the conversion of factor X to Xa, which will form the prothrombinase complex with factor Va, prothrombin (factor II) and calcium, thereby generating thrombin (factor IIa). One of the key functions of thrombin is to convert fibrinogen into fibrin. The tissue factor–factor VIIa complex can also activate factor IX, forming a tenase complex with activated factor IX and factor X, generating additional factor Xa, thereby forming an essential amplification loop. The assembly of the prothrombinase and tenase complex is markedly facilitated if a suitable phospholipid surface is available, ideally presented by activated platelets. In the setting of inflammation-induced activation of coagulation, platelets can be activated directly by endotoxin or by pro-inflammatory mediators, such as platelet activating factor. Thrombin itself is one of the strongest platelet activators in vivo.
Activation of platelets may also accelerate fibrin formation by another mechanism. The expression of TF on monocytes is markedly stimulated by the presence of platelets and granulocytes in a P-selectin-dependent reaction [36]. This effect may be the result of nuclear factor kappa B (NF-κB) activation induced by binding of activated platelets to neutrophils and mononuclear cells [37]. This cellular interaction also markedly enhances the production of Il-1b, Il-8, MCP-1, and TNF-α [38]. The expression of P-selectin on the activated platelet membrane will mediate the adherence of platelets to endothelial cells and leukocytes [39].
Downregulation of physiological anticoagulant and fibrinolytic pathways during inflammation
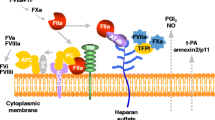
Procoagulant activity is regulated by three important anticoagulant pathways: antithrombin (AT), the protein C system, and tissue factor pathway inhibitor (TFPI). During inflammation-induced activation of coagulation, the function of all three pathways can be impaired [40] (Fig. 1).
Endothelium-associated mediators of coagulation and inflammation. Panel (a) depicts the normal situation in which the endothelium expresses thrombomodulin (TM) (activated by thrombin) and endothelial PC receptor (EPCR), which generate activated PC (APC). Other anticoagulant factors are tissue factor pathway inhibitor (TFPI) and antithrombin (AT) attached to the endothelial surface and from endothelium released tissue-type plasminogen activator (tPA), which promotes fibrinolysis. b Systemic activation of inflammation leads to cytokine release and endothelial perturbation, resulting in release of microparticles (MPs), apoptosis, detachment of endothelial cells, and loss of barrier function. Coagulation is activated by induction of tissue factor (TF) on monocytes, MPs, and endothelium and by release of von Willebrand factor (vWF), which adds to platelet adhesion to the subendothelial surface. Production of glycosaminoglycans (GAGs) is downregulated, and the anticoagulant proteins TFPI, AT, EPCR, and TM are cleaved from the endothelial surface and are impaired in action. Fibrinolysis is impaired as a result of a rise in the main inhibitor of the PA (PAI-1), which outweighs a rise in t-PA, and complement activation is enhanced by loss of activation of thrombin-activatable fibrinolysis inhibitor (TAFI), which normally inhibits complement factor C3a and C5a and bradykinin activity. Anticoagulant proteins in turn modulate cytokine release: tissue factor–factor VIIa (TF-FVIIa), factor (F) Xa, and thrombin exert pro-inflammatory activity by cleaving mainly protease activated receptor (PAR)-1 and PAR-2. APC cleaves PAR-1 in an EPCR-dependent manner and hereby modulates inflammation and apoptosis [27]
The serine protease inhibitor antithrombin is the main inhibitor of thrombin and factor Xa. Without heparin, AT neutralizes coagulation enzymes in a slow, progressive manner [41]. Heparin induces conformational changes in AT that result in at least a 1,000-fold enhancement of AT activity. Thus, the clinical efficacy of heparin is attributed to its interaction with AT. Endogenous glycosaminoglycans, such as heparan sulfates, on the vessel wall also promote AT-mediated inhibition of thrombin and other coagulation enzymes. During severe inflammatory responses, AT levels are markedly decreased owing to impaired synthesis (as a result of a negative acute phase response), degradation by elastase from activated neutrophils, and—quantitatively most importantly—consumption as a consequence of ongoing thrombin generation [42]. Pro-inflammatory cytokines can also cause reduced synthesis of glycosaminoglycans on the endothelial surface, which will also contribute to reduced AT function, since these glycosaminoglycans can act as physiological heparin-like cofactors of AT [43].
Activated protein C (APC) appears to play a central role in the pathogenesis of sepsis and associated organ dysfunction [44]. There is ample evidence that an insufficient functioning of the protein C pathway contributes to the derangement of coagulation in sepsis [45, 46]. In patients with severe inflammation, the protein C system is malfunctioning at virtually all levels. First, plasma levels of the zymogen protein C are low or very low due to impaired synthesis, consumption, and degradation by proteolytic enzymes, such as neutrophil elastase [47–49]. Furthermore, a significant downregulation of thrombomodulin, caused by pro-inflammatory cytokines such as TNF-α and IL-1, has been demonstrated, resulting in diminished protein C activation [50, 51]. Low levels of free protein S may further compromise an adequate function of the protein C system. In plasma, 60% of the co-factor protein S is complexed to a complement regulatory protein, C4b binding protein (C4bBP). Increased plasma levels of C4bBP as a consequence of the acute phase reaction in inflammatory diseases may result in a relative protein S deficiency, which further contributes to a procoagulant state during sepsis. Although it has been shown that the β-chain of C4bBP (which mainly governs the binding to protein S) is largely unaffected during the acute phase response [52], support for this hypothesis comes from studies showing that the infusion of C4bBP in combination with a sublethal dose of Escherichia coli into baboons resulted in a lethal response with severe organ damage due to DIC [53]. Finally, but importantly, in sepsis the EPCR has shown to be downregulated, which may further negatively affect the function of the protein C system [54]. Apart from these effects, sepsis may cause a resistance toward APC by other mechanisms, which are partly dependent on a sharp increase in factor VIII levels (released from endothelial cells), but partly occur by yet unidentified mechanisms [55].
A third inhibitory mechanism of thrombin generation involves TFPI, the main inhibitor of the tissue factor–factor VIIa complex. The role of TFPI in the regulation of inflammation-induced coagulation activation is not completely clear. Experiments showing that administration of recombinant TFPI (and thereby achieving higher than physiological plasma concentrations of TFPI) blocks inflammation-induced thrombin generation in humans, and the observation that pharmacological doses of TFPI are capable of preventing mortality during systemic infection and inflammation suggests that high concentrations of TFPI are capable of importantly modulating tissue factor-mediated coagulation [10, 56].
Central regulators of plasminogen activators and inhibitors during inflammation are TNF-α and IL-1β [57]. Occurrence of these cytokines in the circulation leads to the release of plasminogen activators, in particular tissue-type plasminogen activator (t-PA) and urokinase-type plasminogen activator (u-PA), from storage sites in vascular endothelial cells. However, this increase in plasminogen activation and subsequent plasmin generation is counteracted by a delayed but sustained increase in plasminogen activator inhibitor type 1 (PAI-1) [58]. The resulting effect on fibrinolysis is a complete inhibition and, as a consequence, inadequate fibrin removal, contributing to microvascular thrombosis. Experiments in mice with targeted disruptions of genes encoding components of the plasminogen–plasmin system confirm that fibrinolysis plays a major role in inflammation. Mice with a deficiency of plasminogen activators have more extensive fibrin deposition in organs when challenged with endotoxin, whereas PAI-1 knockout mice, in contrast to wild-type controls, have no microvascular thrombosis upon endotoxin administration [59].
Modulation of inflammation by coagulation in vivo
Communication between inflammation and coagulation is bidirectional, such that coagulation can also modulate inflammatory activity. Coagulation proteases and protease inhibitors not only interact with coagulation protein zymogens but also with specific cell receptors to induce signaling pathways (Fig. 1). In particular, protease interactions that affect inflammatory processes may be important in critically ill patients. In vivo evidence for a role of coagulation–protease stimulation of inflammation comes from experiments showing that the administration of recombinant factor VIIa to healthy human subjects causes a small but significant 3- to 4-fold rise in plasma levels of IL-6 and IL-8 [60].
A pivotal mechanism by which coagulation proteases modulate inflammation is by binding to protease activated receptors or PARs. Four types (PAR 1–4) have been identified, all belonging to the family of transmembrane domain, G-protein-coupled receptors [61]. A typical feature of PARs is that they serve as their own ligand. Proteolytic cleavage by an activated coagulation factor leads to exposure of a neo-amino terminus, which activates the same receptor (and possibly adjacent receptors), initiating transmembrane signaling. PARs are localized in the vasculature on endothelial cells, mononuclear cells, platelets, fibroblasts, and smooth muscle cells [61]. PARs 1, 3, and 4 are thrombin receptors, and PAR-1 can also serve as receptor for the tissue factor–factor VIIa complex and factor Xa. PAR-2 cannot bind thrombin, but can be activated by the tissue factor–factor VIIa complex or factor Xa. Binding of thrombin to its cellular receptor may induce the production of several cytokines and growth factors. Binding of tissue factor–factor VIIa to PAR-2 also results in upregulation of inflammatory responses (production of reactive oxygen species and expression of MHC class II and cell adhesion molecules) in macrophages and was shown to affect neutrophil infiltration and pro-inflammatory cytokine (TNF-α, IL-1β) expression. The in vivo relevance of PARs has been confirmed in various experimental studies using PAR inhibitors or PAR-deficient mice [62–64].
Effects of anticoagulant molecules on inflammation
Antithrombin possesses anti-inflammatory properties, many of which are mediated by its actions in the coagulation cascade [65]. Most importantly, thrombin inhibition by AT blunts activation of many inflammatory mediators. For example, thrombin activates platelets and endothelial cells, which in turn contribute to local inflammation [66]. Activated platelets secrete inflammatory mediators such as IL-1, which stimulate leukocyte activity. In particular, recruitment and adhesion of neutrophils and monocytes to blood vessels within the microcirculation promote inflammation. Increasing evidence suggests that AT possesses potent anti-inflammatory properties independent of its anticoagulation activity [66]. Most of these effects have been demonstrated in vitro or in vivo at high concentrations. Nevertheless, these mechanisms may be important in clinical settings that are driven by a combined activation of inflammation and coagulation. Perhaps most importantly, AT induces prostacyclin release from endothelial cells [67–69]. Prostacyclin inhibits platelet activation and aggregation, blocks neutrophil tethering to blood vessels, and decreases endothelial cell production of various cytokines and chemokines [70]. Additional anti-inflammatory actions of AT are mediated by direct interaction with leukocytes and lymphocytes. Antithrombin binds to receptors, such as syndecan-4, on the cell surfaces of neutrophils, monocytes, and lymphocytes, and blocks the interaction of these cells with endothelial cells [27]. Inhibition of leukocyte–endothelial cell interactions by AT may be mediated by prostacyclin release, downregulation of P-selectin, or prevention of leukocyte activation. Thus, AT directly hinders leukocyte migration and adhesion to endothelial cells, which in turn impacts the severity of capillary leakage and subsequent organ damage. Given the wide-ranging impact of AT on coagulation and inflammation, there are multiple potential clinical applications of AT in different clinical settings that encompass thrombotic states generally not associated with inflammation (e.g., pregnancy) and in coagulation-related disease states with powerful pro-inflammatory elements (e.g., sepsis).
There is compelling evidence that besides their role as an important regulator of coagulation activity components of the protein C system also have an important function in modulating inflammation [71, 72]. APC plays an important role in attenuating the systemic inflammatory response in sepsis as demonstrated in experiments showing that blocking the protein C pathway in septic baboons exacerbated the inflammatory response. In contrast, administration of APC ameliorated the inflammatory activation upon the intravenous infusion of E. coli [13]. Similar experiments in rodents showed identical results and demonstrated a beneficial effect on inflammatory effects in various tissues [73]. Support for the notion that APC has anti-inflammatory properties comes from in vitro observations, demonstrating an APC binding site on monocytes, that may mediate downstream inflammatory processes [74, 75] and from experiments showing that APC can block NF-κB nuclear translocation, which is a prerequisite for increases in pro-inflammatory cytokines and adhesion molecules [76]. These in vitro findings are supported by in vivo studies in mice with targeted disruption of the protein C gene. In these mice with genetic deficiencies of protein C, endotoxemia was associated with a more marked increase in pro-inflammatory cytokines and other inflammatory responses as compared with wild-type mice [77, 78].
It is likely that the effects of APC on inflammation are mediated by the EPCR [71]. Binding of APC to EPCR influences gene expression profiles of cells by inhibiting endotoxin-induced calcium fluxes in the cell and by blocking NF-κB nuclear translocation [75, 76]. Blocking the EPCR with a specific monoclonal antibody aggravates both the coagulation and the inflammatory response to E. coli infusion [54].
Apart from its effect on cytokine levels, APC has been shown to inhibit leukocyte chemotaxis and adhesion of leukocytes to activated endothelium [79, 80]. This notion was confirmed in a hamster endotoxemia model at concentrations of recombinant human APC (rhAPC) that preclude a significant anticoagulant effect [81]. Moreover, in a human model of endotoxin-induced pulmonary inflammation, systemic administration of rhAPC resulted in significant local anti-inflammatory effects [82]. A potential mechanism is that APC inhibits expression of platelet-derived growth factor in the lung [83]. In addition, it has been shown that APC protects against the disruption of endothelial cell barrier in sepsis, probably by interfering with EPCR and PAR-1 on endothelial cells [84–86].
Systemic versus localized responses
Although the mechanisms mentioned above have been demonstrated to occur in vivo as a general response upon pro-inflammatory stimuli, it is likely that marked differences in the procoagulant response as well as the underlying pathogenetic pathway may exist between cells and tissues [87]. This may be caused by differences in cell-specific gene expression, environmental factors, and organ-specific differences. First, localization of coagulation activity may relate to a cell-specific gene expression. For example, inflammatory mediators enhance PAI-1 gene expression in a complex and tissue-specific way [88]. Recent studies have demonstrated that the von Willebrand factor promoter contains cell-specific elements, and similar response elements may be involved in protein synthesis in cells in general [89]. Second, the tissue environment may determine whether specific gene transcription occurs [90]. It is not completely clear why specific sites and organs are at greater risk of developing microvascular thrombosis and also local differences in the consequences of (micro)thrombosis are still poorly understood. Environmental factors underlying the inflammatory response are thought to play a role in this differential coagulative response as well. In mice with disturbances in the plasminogen–plasmin system subjected to hypoxia, the formation of fibrin is induced and is particularly evident in the lungs [59]. In contrast, these same mice respond to endotoxemia with fibrin deposition in the microvasculature of the kidney in particular. Similarly, mice with a functional thrombomodulin deficiency had a marked increase in pulmonary fibrin deposition after hypoxic challenge [91]. In addition, when mice with a functional defect in the thrombomodulin gene were challenged with endotoxin in sublethal amounts, fibrin formation was apparent in the lungs, but not in any other organ studied. In the latter model, fibrin was only temporarily present, and had disappeared after 24 h [92]. These models illustrate the assumption that fibrin formation is a localized phenomenon rather than a generalized process.
Lastly, various organ systems may markedly differ in their endothelial cell response towards inflammation and injury. In general, endothelial cells play a central role in the coagulation response upon systemic inflammation [42]. The endothelium plays a central role in all major pathways involved in the pathogenesis of hemostatic derangement during severe inflammation. Endothelial cells appear to be directly involved in the initiation and regulation of thrombin generation and the inhibition of fibrin removal. Endothelial cells may express tissue factor, which is the main initiator of coagulation. In addition, physiological anticoagulant pathways, such as antithrombin, the protein C system, or tissue factor pathway inhibitor (TFPI), are mostly located on endothelial cells and endothelial cell dysfunction is directly related to impaired regulation of coagulation. Also, endothelial cells are the main storage site of plasminogen activators and inhibitors and can acutely release these factors, thereby importantly mediating fibrinolytic activity or inhibition. Pro-inflammatory cytokines are crucial in mediating these effects on endothelial cells, which themselves may also express cytokines, thereby amplifying the coagulative response [30]. Although not completely clear, various organs may differ in all these endothelial cell-related factors influencing local coagulation activation and fibrin deposition.
Organ-specific responses by endothelial cells
In their excellent overview, Rosenberg and Aird postulate that endothelial cells integrate different extracellular signals and cellular responses in different regions of the vascular bed [89]. Various exogenous stimuli, such as shear stress, inflammatory mediators, and growth factors, exert their action on endothelial cells, and the response of the endothelial cells to transduce the signal may vary between various tissues and even from endothelial cell to endothelial cell within a tissue. As a result, the pro- or anticoagulant response of endothelial cells may differ between organs. Experiments in mice with a targeted deletion of the anticoagulant part of the thrombomodulin gene show abundant fibrin formation in lungs, heart, and spleen [93]. Mice with homozygous deficiencies of plasminogen activators form clots in liver, heart, and lungs, but not in brain or kidneys [94]. Plasminogen activator inhibitor-deficient mice have fibrin deposition predominantly in kidneys [88]. Rosenberg and Aird state that various mechanisms play a role in the individual response of endothelial cells. Cell-to-cell communication may have important effects, as evidenced by the fact that PAI-1 expression in endothelial cells is upregulated if the culture is incubated with medium from aorta or umbilical vein cell culture but, in contrast, is downregulated by addition of conditioned medium from vascular smooth muscle cells [95, 96]. In addition, cell signaling pathways may vary between endothelial cell subtypes. For example, hemodynamic changes may have opposite reactions in nitric oxide mRNA expression in endothelial cells from the aorta or from the pulmonary artery [97]. Another example is provided by the experiment showing that endothelial cells from renal and cerebrovascular vessels have decreased prostacyclin production and more apoptosis when exposed to plasma of patients with thrombocytopenic thrombotic purpura, whereas endothelial cells derived from lungs and liver do not respond to this same stimulus [98]. Lastly, also at the level of transcription a differential phenotype of endothelial cells between various organs can be demonstrated. Coagulation proteins, such as von Willebrand factor, have been shown to respond to various promoters by expressing this factor in different tissues, for example exclusively in heart and skeletal muscle or in brain [90].
Coagulation activation and the kidney
Coagulation is important in two groups of renal disorders in man. In one group, the kidney is the major site of disease, and localized thrombosis and fibrin formation is superimposed on demonstrable immunological and or endothelial damage. These disorders will not be discussed here. In the second group, renal lesions associated with fibrin formation are involved as a consequence of systemic intravascular coagulation or DIC [99]. In the latter group, acute renal failure (ARF) is the usual associated renal presentation, occurring in the course of sepsis, major surgery, severe trauma, and hypovolemic and cardiogenic shock. The pathogenesis of ARF in these conditions is caused by hypoperfusion resulting in ischemia–reperfusion injury. The decrease in oxygen saturation and hormonal dysregulation causes acute tubular necrosis [100]. At least in septic shock, it has been suggested that microthrombi contribute to ARF [101, 102]. The older literature strongly suggests that intravascular coagulation causes immediate changes that are detrimental to renal function. Electron microscopical studies have shown that coagulation causes mesangial swelling and an increase in vacuoles, organelles free ribosomes, and mitochondria [103]. These changes were associated with phagocytosis of fibrin and secretion of basement membrane-like material. The glomerular lesions occurring in the course of DIC may resemble those seen in acute glomerulonephritis, with platelets and fibrin deposits intraluminally, swollen endothelium, subendothelial deposits of fibrin cleavage fragments, and cellular proliferative effects. When these processes continue, complete occlusion of glomerular capillaries and hyalinization of glomeruli may follow [99]. The pathophysiology of renal failure in shock is also thought to be influenced by vasoactive substances, and renal damage was markedly reduced by adrenergic blockade in a model of hemorrhagic shock or endotoxin shock [104]. Catecholamine infusion in experimental animals causes shock and DIC. Heparin reduces the effects of catecholamine-induced shock and endotoxin-related complications in animal models. It thus appears that the combination of hypoperfusion-related ischemia–reperfusion injury and vasoactive reactions are of major influence on the occurrence of ARF in shock. The finding of fibrin deposits suggests that DIC contributes to organ damage, and the observed improvement under heparin treatment supports this concept. The trigger to thrombosis is probably locally induced by the ischemia–reperfusion responses of hypoxia-inducible factor-mediated TF expression [105]. In addition, systemic stimuli such as endotoxin cause cytokine-mediated upregulation of TFmRNA in the kidney, while local fibrinolytic defense mechanisms are also activated (u-PA and t-PA, without concurrent upregulation of PAI-1). Furthermore, experimental studies have demonstrated that specific blockade of the factor VII–TF complex reduced fibrin in the kidney [106]. Infusion of hirudin caused a dose-dependent decrease in mortality and also reduced the amount of fibrin deposition in the kidney. An important role of the protein C system in preventing glomerular thrombosis may be inferred from the abundant presence of thrombomodulin expression on endothelial cells in the glomerulus [107]. In inflammatory glomerular disease, such as acute membranoproliferative or lupus glomerulonephritis, an increase in thrombomodulin expression has been implicated [108]. In contrast, in ischemia–reperfusion injury in kidneys, thrombomodulin has been markedly downregulated. Administration of soluble thrombomodulin to rats with renal ischemia–reperfusion injury prevented massive glomerular thrombosis and kidney dysfunction [109]. In another experimental study of renal ischemia and reperfusion, administration of activated protein C prevented histological changes and the decrease in renal blood flow, and preserved kidney function, whereas treatment with active site-blocked factor Xa, heparin, and inactivated protein C were less effective [110]. It therefore appears that inhibition of coagulation also reduces the amount of fibrin in the kidney. This may imply an improvement of renal function; however, there have been no controlled trials in which the beneficial effect of anticoagulant treatment in patients with DIC and ARF was investigated.
Coagulation activation and the lung
The lungs are among the most frequently affected organs during severe infection and sepsis [111, 112]. Lung injury in this situation is characterized by increased permeability of the alveolar–capillary membrane, diffuse alveolar damage, and the accumulation of pulmonary edema, containing a high concentration of proteases and other proteins. Pathological examination of the injured lung demonstrates epithelial cell injury represented by extensive necrosis of pneumocytes, swelling of endothelial cells with the widening of intercellular junctions, and the formation of hyaline membranes, for an important part composed of fibrin in alveolar ducts and airspaces. At later stages, massive infiltration of neutrophils and other inflammatory cells will occur and fibrin thrombi can be seen in the alveolar capillaries and smaller pulmonary arteries [113]. The abundant presence of intravascular and extravascular fibrin appears to be a specific hallmark of acute lung injury following sepsis and is much more outspoken than the fibrin deposition in other organs. Based on this observation, many authors have hypothesized that fibrin deposition plays an important role in the pathogenesis of acute lung injury in sepsis, a concept that is further supported by large clinical studies in patients with sepsis demonstrating the association between lung injury and coagulation abnormalities [114]. Furthermore, the extensive local fibrin deposition may suggest that local activation of coagulation or perturbation of local physiological regulatory systems could be involved in this. Interestingly, it has been shown that effective blocking of the coagulopathy in experimental sepsis attenuates lung injury and local inflammatory activity, which may point at pivotal cross-talk between the (local) mechanisms of coagulation and inflammation [14, 15].
In BAL fluids from patients with ARDS, it has been demonstrated that there is activation of coagulation and inhibition of fibrinolysis [115–117]. Almost immediately after onset of ARDS, an increased but transient procoagulant activity can be detected in BAL fluid. At the same time, fibrinolytic activity is strongly inhibited and is kept at a low level up to 14 days. Experimental and clinical studies have shown that fibrin deposition is due to tissue factor-mediated thrombin generation and suppressed fibrinolysis [118]. The most important determinants of these local disturbances are TF and PAI-1; high levels of soluble TF can be measured in BAL fluid from patients with ARDS, while increased production of PAI-1 is the most consistent finding reported as being related to suppressed fibrinolytic activity. Recently, lower levels of pulmonary PC levels were correlated with a higher degree of lung injury and worse outcome in patients with acute lung injury [119]. Similar to acute lung injury and ARDS, pneumonia is characterized by a shift in the alveolar hemostatic balance. In BAL fluid from patients with severe pneumonia, a markedly increased procoagulant activity was detected. Concordantly, fibrinolytic activity was depressed in BAL fluids, which is related to high concentrations of PAI-1 in the lungs. Patients at risk for ventilator-associated pneumonia show similar changes in pulmonary fibrin turnover [120]. Similarly, in mechanically ventilated patients who developed pneumonia, an increase in coagulation products was detected in lung lavage fluids. Interestingly, the diagnosis of pneumonia was preceded by a strong increase in PAI-1 levels in the lungs, with a resulting decrease in fibrinolytic activity. Similar to the inflammatory responses in patients with unilateral pneumonia patients, there is overt activation of coagulation and depressed fibrinolytic activity due to PAI-1 upregulation [121]. Recently, it has been demonstrated that the protein C system is also suppressed at the site of infection, contributing to the procoagulant effects of pulmonary infection [121].
Coagulation activation and the intestinal tract
Acute intestinal ischemia and reperfusion may result in impaired intestinal structure and function, in experimental models characterized by intestinal cell swelling and protein leakage and impaired intestinal absorptive capacity. In addition, intra- and extravascular fibrin deposits may be present due to activation of mesenteric coagulation and inhibition of fibrinolysis [122]. Upon 20 to 40 min of occlusion of the superior mesenteric artery and subsequent reperfusion, portal vein plasma levels of thrombin–antithrombin levels increased, indicating local thrombin generation. This increase in portal coagulation activity is associated with a marked fall in protein C activity levels. Simultaneously, markers for fibrinolysis in portal plasma showed a complete inhibition due to an increase in levels of plasminogen activator inhibitor, type 1 (PAI-1). This activation of coagulation upon ischemia–reperfusion could be almost completely blocked by systemic administration of activated protein C, whereas heparin and antithrombin were less effective (Schoots et al., submitted for publication). Interestingly, amelioration of ischemia–reperfusion-induced intestinal intra- and extravascular fibrin deposition by administration of activated protein C caused a significant improvement in intestinal function.
Coagulation activation and the liver
The liver is the major site of synthesis of almost all coagulation factors. In addition, Kupfer cells of the liver are most important bacterial scavengers, and neutralize bacterial products and pro-inflammatory cytokines. Impaired synthesis of physiological anticoagulant proteins antithrombin and protein C, and low levels of free protein S due to acute phase upregulation of C4b-binding protein (the carrier of protein S) are well-known consequences of impaired liver function [123]. However, failure of the coagulation system is not only a consequence of liver failure but may also contribute to the pathogenesis of liver failure in systemic inflammatory states. Under these circumstances, endothelial cells of the liver show a marked upregulation of tissue factor, leading to local thrombin generation and fibrinogen to fibrin conversion [124]. A marked cross-talk between coagulation and inflammation is also strongly present in liver tissue, as protease-activated receptors (PARs) are abundantly present and activated coagulation proteases may not only lead to fibrin formation but also to increased inflammation, and in case of liver tissue, ultimately to tissue fibrosis [125]. Indeed, it has been shown that anticoagulant treatment can prevent ischemia–reperfusion injury in an experimental model in rat livers [126].
Conclusion
The response of the coagulation system upon systemic inflammation may considerably vary between cells, tissues, and organs. This may explain, in part, the variable clinical presentation of multiple organ failure in patients with a systemic inflammatory response upon sepsis or trauma. Many coagulation pathways in various organs may act according to parallel routes, but marked differences exist in the emphasis of a specific mechanism in a specific organ system. Detailed knowledge on the site-specific activation and regulation of coagulation may provide more insight in better management strategies in case of specific organ failures in the setting of a systemic inflammatory response.
References
Bauer KA, Rosenberg RD (1987) The pathophysiology of the prethrombotic state in humans: insights gained from studies using markers of hemostatic system activation. Blood 70(2):343–350
Levi M, ten Cate H (1999) Disseminated intravascular coagulation. N Engl J Med 341(8):586–592
Levi M, Schultz M, van der Poll T (2011) Coagulation biomarkers in critically ill patients. Crit Care Clin 27(2):281–297
Levi M (2010) Disseminated intravascular coagulation: a disease-specific approach. Semin Thromb Hemost 36(4):363–365
Levi M (2004) Current understanding of disseminated intravascular coagulation. Br J Haematol 124(5):567–576
Levi M, Keller TT, van Gorp E, ten Cate H (2003) Infection and inflammation and the coagulation system. Cardiovasc Res 60(1):26–39
Robboy SJ, Major MC, Colman RW, Minna JD (1972) Pathology of disseminated intravascular coagulation (DIC). Analysis of 26 cases. Hum Pathol 3(3):327–343
Shimamura K, Oka K, Nakazawa M, Kojima M (1983) Distribution patterns of microthrombi in disseminated intravascular coagulation. Arch Pathol Lab Med 107(10):543–547
Coalson JJ (1986) Pathology of sepsis, septic shock, and multiple organ failure. In: Sibbald WJ, Sprung CL (eds) Perspective on sepsis and septic shock. Society of Critical Care Medicine, Fullerton, pp 27–59
Creasey AA, Chang AC, Feigen L, Wun TC, Taylor FBJ, Hinshaw LB (1993) Tissue factor pathway inhibitor reduces mortality from Escherichia coli septic shock. J Clin Invest 91(6):2850–2856
Kessler CM, Tang Z, Jacobs HM, Szymanski LM (1997) The suprapharmacologic dosing of antithrombin concentrate for Staphylococcus aureus-induced disseminated intravascular coagulation in guinea pigs: substantial reduction in mortality and morbidity. Blood 89(12):4393–4401
Taylor FBJ, Chang A, Ruf W, Morrissey JH, Hinshaw L, Catlett R, Blick K, Edgington TS (1991) Lethal E. coli septic shock is prevented by blocking tissue factor with monoclonal antibody. Circ Shock 33(3):127–134
Taylor FBJ, Chang A, Esmon CT, D'Angelo A, Vigano-D'Angelo S, Blick KE (1987) Protein C prevents the coagulopathic and lethal effects of Escherichia coli infusion in the baboon. J Clin Invest 79(3):918–925
Welty-Wolf KE, Carraway MS, Miller DL, Ortel TL, Ezban M, Ghio AJ, Idell S, Piantadosi CA (2001) Coagulation blockade prevents sepsis-induced respiratory and renal failure in baboons. Am J Respir Crit Care Med 164(10 Pt 1):1988–1996
Miller DL, Welty-Wolf K, Carraway MS, Ezban M, Ghio A, Suliman H, Piantadosi CA (2002) Extrinsic coagulation blockade attenuates lung injury and proinflammatory cytokine release after intratracheal lipopolysaccharide. Am J Respir Cell Mol Biol 26(6):650–658
Fourrier F, Chopin C, Goudemand J, Hendrycx S, Caron C, Rime A, Marey A, Lestavel P (1992) Septic shock, multiple organ failure, and disseminated intravascular coagulation. Compared patterns of antithrombin III, protein C, and protein S deficiencies [see comments]. Chest 101(3):816–823
Dhainaut JF, Yan SB, Joyce DE, Pettila V, Basson BR, Brandt JT, Sundin D, Levi M (2004) Treatment effects of drotrecogin alfa (activated) in patients with severe sepsis with or without overt disseminated intravascular coagulation. J Thromb Haemost 2:1924–1933
Levi M, Lowenberg EC (2008) Thrombocytopenia in critically ill patients. Semin Thromb Hemost 34(5):417–424
Vanderschueren S, De Weerdt A, Malbrain M, Vankersschaever D, Frans E, Wilmer A, Bobbaers H (2000) Thrombocytopenia and prognosis in intensive care. Crit Care Med 28(6):1871–1876
Levi MM, Eerenberg E, Lowenberg E, Kamphuisen PW (2010) Bleeding in patients using new anticoagulants or antiplatelet agents: risk factors and management. Neth J Med 68(2):68–76
Oppenheim-Eden A, Glantz L, Eidelman LA, Sprung CL (1999) Spontaneous intracerebral hemorrhage in critically ill patients: incidence over six years and associated factors. Intensive Care Med 25(1):63–67
Strauss R, Wehler M, Mehler K, Kreutzer D, Koebnick C, Hahn EG (2002) Thrombocytopenia in patients in the medical intensive care unit: bleeding prevalence, transfusion requirements, and outcome. Crit Care Med 30(8):1765–1771
Stephan F, Hollande J, Richard O, Cheffi A, Maier-Redelsperger M, Flahault A (1999) Thrombocytopenia in a surgical ICU. Chest 115(5):1363–1370
Akca S, Haji Michael P, de Medonca A, Suter PM, Levi M, Vincent JL (2002) The time course of platelet counts in critically ill patients. Crit Care Med 30:753–756
Chakraverty R, Davidson S, Peggs K, Stross P, Garrard C, Littlewood TJ (1996) The incidence and cause of coagulopathies in an intensive care population. Br J Haematol 93(2):460–463
MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M (2003) Early coagulopathy predicts mortality in trauma. J Trauma 55(1):39–44
Levi M, van der Poll T (2010) Inflammation and coagulation. Crit Care Med 38(2 Suppl):S26–S34
Levi M, ten Cate H, Bauer KA, van der Poll T, Edgington TS, Buller HR, van Deventer SJ, Hack CE, ten Cate JW, Rosenberg RD (1994) Inhibition of endotoxin-induced activation of coagulation and fibrinolysis by pentoxifylline or by a monoclonal anti-tissue factor antibody in chimpanzees. J Clin Invest 93(1):114–120
van der Poll T, Levi M, Hack CE, ten Cate H, van Deventer SJ, Eerenberg AJ, de Groot E, Jansen J, Gallati H, Buller HR (1994) Elimination of interleukin 6 attenuates coagulation activation in experimental endotoxemia in chimpanzees. J Exp Med 179(4):1253–1259
Levi M, van der Poll T, ten Cate H, van Deventer SJ (1997) The cytokine-mediated imbalance between coagulant and anticoagulant mechanisms in sepsis and endotoxaemia. Eur J Clin Invest 27(1):3–9
Libby P, Aikawa M (2002) Stabilization of atherosclerotic plaques: new mechanisms and clinical targets. Nat Med 8(11):1257–1262
Osterud B, Rao LV, Olsen JO (2000) Induction of tissue factor expression in whole blood—lack of evidence for the presence of tissue factor expression on granulocytes. Thromb Haemost 83:861–867
Franco RF, de Jonge E, Dekkers PE, Timmerman JJ, Spek CA, van Deventer SJ, van der Poll T, Ten Cate H, Reitsma PH (2000) The in vivo kinetics of tissue factor messenger RNA expression during human endotoxemia: relationship with activation of coagulation. Blood 96(2):554–559
Rauch U, Bonderman D, Bohrmann B, Badimon JJ, Himber J, Riederer MA, Nemerson Y (2000) Transfer of tissue factor from leukocytes to platelets is mediated by CD15 and tissue factor. Blood 96(1):170–175
Osterud B, Bjorklid E (2006) Sources of tissue factor. Semin Thromb Hemost 32(1):11–23
Osterud B (1998) Tissue factor expression by monocytes: regulation and pathophysiological roles. Blood Coagul Fibrinolysis 9(Suppl 1):S9–S14
Furie B, Furie BC (2004) Role of platelet P-selectin and microparticle PSGL-1 in thrombus formation. Trends Mol Med 10(4):171–178
Neumann FJ, Marx N, Gawaz M, Brand K, Ott I, Rokitta C, Sticherling MC, May A, Schomig A (1997) Induction of cytokine expression in leukocytes by binding of thrombin-stimulated platelets. Circulation 95(10):2387–2394
Lowenberg EC, Meijers JC, Levi M (2010) Platelet–vessel wall interaction in health and disease. Neth J Med 68(6):242–251
Levi M, van der Poll T (2008) The role of natural anticoagulants in the pathogenesis and management of systemic activation of coagulation and inflammation in critically ill patients. Semin Thromb Hemost 34(5):459–468
Levi M (2005) Antithrombin in sepsis revisited. Crit Care 9(6):624–625
Levi M, van der Poll T, Buller HR (2004) Bidirectional relation between inflammation and coagulation. Circulation 109(22):2698–2704
Kobayashi M, Shimada K, Ozawa T (1990) Human recombinant interleukin-1 beta- and tumor necrosis factor alpha-mediated suppression of heparin-like compounds on cultured porcine aortic endothelial cells. J Cell Physiol 144(3):383–390
Levi M, van der Poll T (2007) Recombinant human activated protein C: current insights into its mechanism of action. Crit Care 11(Suppl 5):S3
Esmon CT (2001) Role of coagulation inhibitors in inflammation. Thromb Haemost 86(1):51–56
Levi M, de Jonge E, van der Poll T (2001) Rationale for restoration of physiological anticoagulant pathways in patients with sepsis and disseminated intravascular coagulation. Crit Care Med 29(7 Suppl):S90–S94
Mesters RM, Helterbrand J, Utterback BG, Yan B, Chao YB, Fernandez JA, Griffin JH, Hartman DL (2000) Prognostic value of protein C concentrations in neutropenic patients at high risk of severe septic complications. Crit Care Med 28(7):2209–2216
Vary TC, Kimball SR (1992) Regulation of hepatic protein synthesis in chronic inflammation and sepsis. Am J Physiol 262(2 Pt 1):C445–C452
Eckle I, Seitz R, Egbring R, Kolb G, Havemann K (1991) Protein C degradation in vitro by neutrophil elastase. Biol Chem Hoppe Seyler 372(11):1007–1013
Nawroth PP, Stern DM (1986) Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med 163(3):740–745
Faust SN, Levin M, Harrison OB, Goldin RD, Lockhart MS, Kondaveeti S, Laszik Z, Esmon CT, Heyderman RS (2001) Dysfunction of endothelial protein C activation in severe meningococcal sepsis. N Engl J Med 345(6):408–416
Garcia de Frutos P, Alim RI, Hardig Y, Zoller B, Dahlback B (1994) Differential regulation of alpha and beta chains of C4b-binding protein during acute-phase response resulting in stable plasma levels of free anticoagulant protein S. Blood 84(3):815–822
Taylor FBJ, Dahlback B, Chang AC, Lockhart MS, Hatanaka K, Peer G, Esmon CT (1995) Role of free protein S and C4b binding protein in regulating the coagulant response to Escherichia coli. Blood 86(7):2642–2652
Taylor FBJ, Stearns-Kurosawa DJ, Kurosawa S, Ferrell G, Chang AC, Laszik Z, Kosanke S, Peer G, Esmon CT (2000) The endothelial cell protein C receptor aids in host defense against Escherichia coli sepsis. Blood 95(5):1680–1686
De Pont AC, Bakhtiari K, Hutten BA, de Jonge E, Vlasuk GP, Rote WE, Levi M, Buller HR, Meijers JC (2006) Endotoxaemia induces resistance to activated protein C in healthy humans. Br J Haematol 134(2):213–219
de Jonge E, Dekkers PE, Creasey AA, Hack CE, Paulson SK, Karim A, Kesecioglu J, Levi M, van Deventer SJ, van der Poll T (2000) Tissue factor pathway inhibitor (TFPI) dose-dependently inhibits coagulation activtion without influencing the fibrinolytic and cytokine response during human endotoxemia. Blood 95:1124–1129
Biemond BJ, Levi M, ten Cate H, van der Poll T, Buller HR, Hack CE, ten Cate JW (1995) Plasminogen activator and plasminogen activator inhibitor I release during experimental endotoxaemia in chimpanzees: effect of interventions in the cytokine and coagulation cascades. Clin Sci (Colch) 88(5):587–594
van der Poll T, Levi M, Buller HR, van Deventer SJ, de Boer JP, Hack CE, ten Cate JW (1991) Fibrinolytic response to tumor necrosis factor in healthy subjects. J Exp Med 174(3):729–732
Yamamoto K, Loskutoff DJ (1996) Fibrin deposition in tissues from endotoxin-treated mice correlates with decreases in the expression of urokinase-type but not tissue-type plasminogen activator. J Clin Invest 97(11):2440–2451
de Jonge E, Friederich PW, Levi M, van der Poll T (2003) Activation of coagulation by administration of recombinant factor VIIa elcicits interleukin-6 and interleukin-8 release in healthy humen subjects. Clin Diagn Lab Immunol 10:495–497
Coughlin SR (2000) Thrombin signalling and protease-activated receptors. Nature 407(6801):258–264
Camerer E, Cornelissen I, Kataoka H, Duong DN, Zheng YW, Coughlin SR (2006) Roles of protease-activated receptors in a mouse model of endotoxemia. Blood 107(10):3912–3921
Slofstra SH, Bijlsma MF, Groot AP, Reitsma PH, Lindhout T, ten Cate CH, Spek CA (2007) Protease-activated receptor-4 inhibition protects from multiorgan failure in a murine model of systemic inflammation. Blood 110(9):3176–3182
Sevastos J, Kennedy SE, Davis DR, Sam M, Peake PW, Charlesworth JA, Mackman N, Erlich JH (2007) Tissue factor deficiency and PAR-1 deficiency are protective against renal ischemia reperfusion injury. Blood 109(2):577–583
Roemisch J, Gray E, Hoffmann JN, Wiedermann CJ (2002) Antithrombin: a new look at the actions of a serine protease inhibitor. Blood Coagul Fibrinol 13(8):657–670
Opal SM (2003) Interactions between coagulation and inflammation. Scand J Infect Dis 35(9):545–554
Harada N, Okajima K, Kushimoto S, Isobe H, Tanaka K (1999) Antithrombin reduces ischemia/reperfusion injury of rat liver by increasing the hepatic level of prostacyclin. Blood 93(1):157–164
Horie S, Ishii H, Kazama M (1990) Heparin-like glycosaminoglycan is a receptor for antithrombin III-dependent but not for thrombin-dependent prostacyclin production in human endothelial cells. Thromb Res 59(6):895–904
Mizutani A, Okajima K, Uchiba M, Isobe H, Harada N, Mizutani S, Noguchi T (2003) Antithrombin reduces ischemia/reperfusion-induced renal injury in rats by inhibiting leukocyte activation through promotion of prostacyclin production. Blood 101(8):3029–3036
Uchiba M, Okajima K, Murakami K (1998) Effects of various doses of antithrombin III on endotoxin-induced endothelial cell injury and coagulation abnormalities in rats. Thromb Res 89(5):233–241
Esmon CT (2002) New mechanisms for vascular control of inflammation mediated by natural anticoagulant proteins. J Exp Med 196(5):561–564
Okajima K (2001) Regulation of inflammatory responses by natural anticoagulants. Immunol Rev 184:258–274
Murakami K, Okajima K, Uchiba M, Johno M, Nakagaki T, Okabe H, Takatsuki K (1996) Activated protein C attenuates endotoxin-induced pulmonary vascular injury by inhibiting activated leukocytes in rats. Blood 87(2):642–647
Hancock WW, Tsuchida A, Hau H, Thomson NM, Salem HH (1992) The anticoagulants protein C and protein S display potent antiinflammatory and immunosuppressive effects relevant to transplant biology and therapy. Transplant Proc 24(5):2302–2303
Hancock WW, Grey ST, Hau L, Akalin E, Orthner C, Sayegh MH, Salem HH (1995) Binding of activated protein C to a specific receptor on human mononuclear phagocytes inhibits intracellular calcium signaling and monocyte-dependent proliferative responses. Transplantation 60(12):1525–1532
White B, Schmidt M, Murphy C, Livingstone W, O'Toole D, Lawler M, O'Neill L, Kelleher D, Schwarz HP, Smith OP (2000) Activated protein C inhibits lipopolysaccharide-induced nuclear translocation of nuclear factor kappaB (NF-kappaB) and tumour necrosis factor alpha (TNF-alpha) production in the THP-1 monocytic cell line. Br J Haematol 110(1):130–134
Levi M, Dorffler-Melly J, Reitsma PH, Buller HR, Florquin S, van der Poll T, Carmeliet P (2003) Aggravation of endotoxin-induced disseminated intravascular coagulation and cytokine activation in heterozygous protein C deficient mice. Blood 101:4823–4827
Lay AJ, Donahue D, Tsai MJ, Castellino FJ (2007) Acute inflammation is exacerbated in mice genetically predisposed to a severe protein C deficiency. Blood 109(5):1984–1991
Feistritzer C, Sturn DH, Kaneider NC, Djanani A, Wiedermann CJ (2003) Endothelial protein C receptor-dependent inhibition of human eosinophil chemotaxis by protein C. J Allergy Clin Immunol 112(2):375–381
Sturn DH, Kaneider NC, Feistritzer C, Djanani A, Fukudome K, Wiedermann CJ (2003) Expression and function of the endothelial protein C receptor in human neutrophils. Blood 102(4):1499–1505
Hoffmann JN, Vollmar B, Laschke MW, Inthorn D, Fertmann J, Schildberg FW, Menger MD (2004) Microhemodynamic and cellular mechanisms of activated protein C action during endotoxemia. Crit Care Med 32(4):1011–1017
Nick JA, Coldren CD, Geraci MW, Poch KR, Fouty BW, O'Brien J, Gruber M, Zarini S, Murphy RC, Kuhn K, Richter D, Kast KR, Abraham E (2004) Recombinant human activated protein C reduces human endotoxin-induced pulmonary inflammation via inhibition of neutrophil chemotaxis. Blood 104(13):3878–3885
Shimizu S, Gabazza EC, Taguchi O, Yasui H, Taguchi Y, Hayashi T, Ido M, Shimizu T, Nakagaki T, Kobayashi H, Fukudome K, Tsuneyoshi N, Essandro-Gabazza CN, Izumizaki M, Iwase M, Homma I, Adachi Y, Suzuki K (2003) Activated protein C inhibits the expression of platelet-derived growth factor in the lung. Am J Respir Crit Care Med 167(10):1416–1426
Zeng W, Matter WF, Yan SB, Um SL, Vlahos CJ, Liu L (2004) Effect of drotrecogin alfa (activated) on human endothelial cell permeability and Rho kinase signaling. Crit Care Med 32(5 Suppl):S302–S308
Feistritzer C, Riewald M (2005) Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood 105(8):3178–3184
Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR, Camp SM, Ye SQ, Garcia JG (2005) Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J Biol Chem 280(17):17286–17293
Aird WC (2001) Vascular bed-specific hemostasis: role of endothelium in sepsis pathogenesis. Crit Care Med 29(7 Suppl):S28–S34
Sawdey MS, Loskutoff DJ (1991) Regulation of murine type 1 plasminogen activator inhibitor gene expression in vivo. Tissue specificity and induction by lipopolysaccharide, tumor necrosis factor-alpha, and transforming growth factor-beta. J Clin Invest 88(4):1346–1353
Rosenberg RD, Aird WC (1999) Vascular-bed-specific hemostasis and hypercoagulable states. N Engl J Med 340(20):1555–1564
Aird WC, Edelberg JM, Weiler-Guettler H, Simmons WW, Smith TW, Rosenberg RD (1997) Vascular bed-specific expression of an endothelial cell gene is programmed by the tissue microenvironment. J Cell Biol 138(5):1117–1124
Healy AM, Hancock WW, Christie PD, Rayburn HB, Rosenberg RD (1998) Intravascular coagulation activation in a murine model of thrombomodulin deficiency: effects of lesion size, age, and hypoxia on fibrin deposition. Blood 92(11):4188–4197
ten Cate H (2000) Pathophysiology of disseminated intravascular coagulation in sepsis. Crit Care Med 28:S9–S11
Weiler H, Lindner V, Kerlin B, Isermann BH, Hendrickson SB, Cooley BC, Meh DA, Mosesson MW, Shworak NW, Post MJ, Conway EM, Ulfman LH, Von AU, Weitz JI (2001) Characterization of a mouse model for thrombomodulin deficiency. Arterioscler Thromb Vasc Biol 21(9):1531–1537
Pinsky DJ, Liao H, Lawson CA, Yan SF, Chen J, Carmeliet P, Loskutoff DJ, Stern DM (1998) Coordinated induction of plasminogen activator inhibitor-1 (PAI-1) and inhibition of plasminogen activator gene expression by hypoxia promotes pulmonary vascular fibrin deposition. J Clin Invest 102(5):919–928
Christ G, Seiffert D, Hufnagl P, Gessl A, Wojta J, Binder BR (1993) Type 1 plasminogen activator inhibitor synthesis of endothelial cells is downregulated by smooth muscle cells. Blood 81(5):1277–1283
Gallicchio M, Argyriou S, Ianches G, Filonzi EL, Zoellner H, Hamilton JA, McGrath K, Wojta J (1994) Stimulation of PAI-1 expression in endothelial cells by cultured vascular smooth muscle cells. Arterioscler Thromb 14(5):815–823
Everett AD, Le Cras TD, Xue C, Johns RA (1998) eNOS expression is not altered in pulmonary vascular remodeling due to increased pulmonary blood flow. Am J Physiol 274(6 Pt 1):L1058–L1065
Mitra D, Jaffe EA, Weksler B, Hajjar KA, Soderland C, Laurence J (1997) Thrombotic thrombocytopenic purpura and sporadic hemolytic–uremic syndrome plasmas induce apoptosis in restricted lineages of human microvascular endothelial cells. Blood 89(4):1224–1234
Kincaid-Smith P (1972) Coagulation and renal disease. Kidney Int 2(4):183–190 [Review] [99 refs]
Conlon PJ, Kovalik E, Schwab SJ (1995) Percutaneous renal biopsy of ventilated intensive care unit patients. Clin Nephrol 43(5):309–311
Davenport A (1997) The coagulation system in the critically ill patient with acute renal failure and the effect of an extracorporeal circuit. Am J Kidney Dis 30(5 Suppl 4):S20–S27 [Review] [26 refs]
Kanfer A (1992) Glomerular coagulation system in renal diseases. Ren Fail 14(3):407–412
Vassali P, Simon G, Rouiller C (1963) Electron microscopic study of glomerular lesions resulting from intravascular fibrin formation. Am J Pathol 43:579–617
Grandchamp A, Ayer G, Truniger B (1971) Pathogenesis of redistribution of intrarenal blood flow in haemorrhagic hypotension. Eur J Clin Invest 1(4):271–276
O'Rourke JF, Pugh CW, Bartlett SM, Ratcliffe PJ (1996) Identification of hypoxically inducible mRNAs in HeLa cells using differential-display PCR. Role of hypoxia-inducible factor-1. Eur J Biochem 241(2):403–410
Yamazaki M, Asakura H, Aoshima K, Saito M, Jokaji H, Uotani C, Kumabashiri I, Morishita E, Ikeda T, Matsuda T (1994) Effects of DX-9065a, an orally active, newly synthesized and specific inhibitor of factor Xa, against experimental disseminated intravascular coagulation in rats. Thromb Haemost 72(3):392–396
Terada Y, Eguchi Y, Nosaka S, Toba T, Nakamura T, Shimizu Y (2003) Capillary endothelial thrombomodulin expression and fibrin deposition in rats with continuous and bolus lipopolysaccharide administration. Lab Invest 83(8):1165–1173
Mizutani M, Yuzawa Y, Maruyama I, Sakamoto N, Matsuo S (1993) Glomerular localization of thrombomodulin in human glomerulonephritis. Lab Invest 69(2):193–202
Ikeguchi H, Maruyama S, Morita Y, Fujita Y, Kato T, Natori Y, Akatsu H, Campbell W, Okada N, Okada H, Yuzawa Y, Matsuo S (2002) Effects of human soluble thrombomodulin on experimental glomerulonephritis. Kidney Int 61(2):490–501
Mizutani A, Okajima K, Uchiba M, Noguchi T (2000) Activated protein C reduces ischemia/reperfusion-induced renal injury in rats by inhibiting leukocyte activation. Blood 95(12):3781–3787
Idell S (1994) Extravascular coagulation and fibrin deposition in acute lung injury. New Horizons 2(4):566–574
Martin GS, Bernard GR (2001) Airway and lung in sepsis. Intensive Care Med 27(Suppl 1):S63–S79
Bellingan GJ (2002) The pulmonary physician in critical care * 6: the pathogenesis of ALI/ARDS. Thorax 57(6):540–546
Welty-Wolf KE, Carraway MS, Ortel TL, Piantadosi CA (2002) Coagulation and inflammation in acute lung injury. Thromb Haemost 88(1):17–25
Bertozzi P, Astedt B, Zenzius L, Lynch K, LeMaire F, Zapol W, Chapman HA Jr (1990) Depressed bronchoalveolar urokinase activity in patients with adult respiratory distress syndrome. N Engl J Med 322(13):890–897
Idell S (1994) Extravascular coagulation and fibrin deposition in acute lung injury. New Horiz 2(4):566–574
Fuchs-Buder T, de Moerloose P, Ricou B, Reber G, Vifian C, Nicod L, Romand JA, Suter PM (1996) Time course of procoagulant activity and D dimer in bronchoalveolar fluid of patients at risk for or with acute respiratory distress syndrome. Am J Respir Crit Care Med 153(1):163–167
Levi M, van der Poll T, ten Cate H, Kuipers B, Biemond BJ, Jansen HM, ten Cate JW (1998) Differential effects of anti-cytokine treatment on bronchoalveolar hemostasis in endotoxemic chimpanzees. Am J Respir Crit Care Med 158(1):92–98
Ware LB, Fang X, Matthay MA (2003) Protein C and thrombomodulin in human acute lung injury. Am J Physiol Lung Cell Mol Physiol 285(3):L514–L521
Schultz MJ, Millo J, Levi M, Hack CE, Weverling GJ, Garrard CS, van der Poll T (2004) Local activation of coagulation and inhibition of fibrinolysis in the lung during ventilator associated pneumonia. Thorax 59(2):130–135
Choi G, Schultz MJ, van Till JW, Bresser P, Van Der Zee JS, Boermeester MA, Levi M, van der Poll T (2004) Disturbed alveolar fibrin turnover during pneumonia is restricted to the site of infection. Eur Respir J 24:786–789
Schoots IG, Levi M, Roossink EH, Bijlsma PB, Van Gulik TM (2003) Local intravascular coagulation and fibrin deposition on intestinal ischemia–reperfusion in rats. Surgery 133(4):411–419
Dhainaut JF, Marin N, Mignon A, Vinsonneau C (2001) Hepatic response to sepsis: interaction between coagulation and inflammatory processes. Crit Care Med 29(7 Suppl):S42–S47
Kobayashi Y, Yoshimura N, Yamagishi H, Oka T (1998) Role of tissue factor in ischemic reperfusion injury: (I). Tissue factor levels of liver tissue and serum after hepatic injury in rats. Transplant Proc 30(7):3726–3727
Chambers RC, Laurent GJ (2002) Coagulation cascade proteases and tissue fibrosis. Biochem Soc Trans 30(2):194–200
Hisama N, Yamaguchi Y, Okajima K, Uchiba M, Murakami K, Mori K, Yamada S, Ogawa M (1996) Anticoagulant pretreatment attenuates production of cytokine-induced neutrophil chemoattractant following ischemia–reperfusion of rat liver. Dig Dis Sci 41(7):1481–1486
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published as part of the Special Issue on Coagulation & Inflammation [34:1]
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Levi, M., van der Poll, T. & Schultz, M. Systemic versus localized coagulation activation contributing to organ failure in critically ill patients. Semin Immunopathol 34, 167–179 (2012). https://doi.org/10.1007/s00281-011-0283-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-011-0283-7