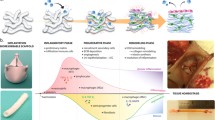
Abstract
Tissue engineering aims at the creation of living neo-tissues identical or close to their native human counterparts. As basis of this approach, temporary biodegradable supporter matrices are fabricated in the shape of a desired construct, which promote tissue strength and provide functionality until sufficient neo-tissue is formed. Besides fully synthetic polymer-based scaffolds, decellularized biological tissue of xenogenic or homogenic origin can be used. In a second step, these scaffolds are seeded with autologous cells attaching to the scaffold microstructure. In order to promote neo-tissue formation and maturation, the seeded scaffolds are exposed to different forms of stimulation. In cardiovascular tissue engineering, this “conditioning” can be achieved via culture media and biomimetic in vitro exposure, e.g., using flow bioreactors. This aims at adequate cellular differentiation, proliferation, and extracellular matrix production to form a living tissue called the construct. These living autologous constructs, such as heart valves or vascular grafts, are created in vitro, comprising a viable interstitium with repair and remodeling capabilities already prior to implantation. In situ further in vivo remodeling is intended to recapitulate physiological vascular architecture and function. The remodeling mechanisms were shown to be dominated by monocytic infiltration and chemotactic host-cell attraction leading into a multifaceted inflammatory process and neo-tissue formation. Key molecules of these processes can be integrated into the scaffold matrix to direct cell and tissue fate in vivo.



Similar content being viewed by others
References
Schoen FJ (2008) Evolving concepts of cardiac valve dynamics: the continuum of development, functional structure, pathobiology, and tissue engineering. Circulation 118:1864–1880
Yoganathan AP, He Z et al (2004) Fluid mechanics of heart valves. Annu Rev Biomed Eng 6:331–362
Dasi LP, Simon HA, Sucosky P et al (2009) Fluid mechanics of artificial heart valves. Clin Exp Pharmacol Physiol 36:225–237
Yacoub MH, Takkenberg JJ (2005) Will heart valve tissue engineering change the world? Nat Clin Pract Cardiovasc Med 2:60–61
Zilla P, Brink J, Human P et al (2008) Prosthetic heart valves: catering for the few. Biomaterials 29:385–406
Langer R, Vacanti JP (1993) Tissue engineering. Science 260:920–926
Sutherland FW, Perry TE, Yu Y, Sherwood MC, Rabkin E, Masuda Y, Garcia GA, McLellan DL, Engelmayr GC Jr, Sacks MS, Schoen FJ, Mayer JE Jr (2005) From stem cells to viable autologous semilunar heart valve. Circulation 31(111):2783–2791
Schmidt D, Achermann J, Odermatt B et al (2007) Prenatally fabricated autologous human living heart valves based on amniotic fluid derived progenitor cells as single cell source. Circulation 116:I64–I70
Schmidt D, Mol A, Breymann C et al (2006) Living autologous heart valves engineered from human prenatally harvested progenitors. Circulation 4(114):I125–I131
Matheny RG, Hutchison ML, Dryden PE et al (2000) Porcine small intestine submucosa as a pulmonary valve leaflet substitute. J Heart Valve Dis 9:769–775
Brennan MP, Dardik A, Hibino N, Roh JD, Nelson GN, Papademitris X, Shinoka T, Breuer CK (2008) Tissue-engineered vascular grafts demonstrate evidence of growth and development when implanted in a juvenile animal model. Ann Surg 248:370–377
Schmidt D, Stock UA, Hoerstrup SP (2007) Tissue engineering of heart valves using decellularized xenogeneic or polymeric starter matrices. Philos Trans R Soc Lond B Biol Sci 29(362):1505–1512
Brody S, Pandit A (2007) Approaches to heart valve tissue engineering scaffold design. J Biomed Mater Res B Appl Biomater 83:16–43
Sacks MS, Schoen FJ, Mayer JE (2009) Bioengineering challenges for heart valve tissue engineering. Annu Rev Biomed Eng 11:289–313
Schmidt D, Hoerstrup SP (2007) Tissue engineered heart valves based on human cells. Swiss Med Wkly 155:80–85
Shin’oka T, Ma PX, Shum-Tim D et al (1996) Tissue-engineered heart valves. Autologous valve leaflet replacement study in a lamb model. Circulation 1(94):II164–II168
Shin'oka T, Shum-Tim D, Ma PX (1997) Tissue-engineered heart valve leaflets: does cell origin affect outcome? Circulation 96:II-102–II-107
Hoerstrup SP, Zund G, Schoeberlein A et al (1998) Fluorescence activated cell sorting: a reliable method in tissue engineering of a bioprosthetic heart valve. Ann Thorac Surg 66:1653–1657
Hoerstrup SP, Sodian R, Daebritz S et al (2000) Functional living trileaflet heart valves grown in vitro. Circulation 102:III44–III49
Shin'oka T, Breuer CK, Tanel RE (1995) Tissue engineering heart valves: valve leaflet replacement study in a lamb model. Ann Thorac Surg 60:513–516
Sodian R, Hoerstrup SP, Sperling JS et al (2000) Early in vivo experience with tissue-engineered trileaflet heart valves. Circulation 102:22–29
Zund G, Hoerstrup SP, Schoeberlein A et al (1998) Tissue engineering: a new approach in cardiovascular surgery: seeding of human fibroblasts followed by human endothelial cells on resorbable mesh. Eur J Cardiothorac Surg 13:160–164
Schnell AM, Hoerstrup SP, Zund G et al (2001) Optimal cell source for cardiovascular tissue engineering: venous vs. aortic human myofibroblasts. Thorac Cardiovasc Surg 49:221–225
Kadner A, Hoerstrup SP, Zund G et al (2002) A new source for cardiovascular tissue engineering: human bone marrow stromal cells. Eur J Cardiothorac Surg 21:1055–1060
Perry TE, Kaushal S, Sutherland FW et al (2003) Thoracic surgery directors association award. Bone marrow as a cell source for tissue engineering heart valves. Ann Thorac Surg 75:761–767
Hoerstrup SP, Kadner A, Melnitchouk S et al (2002) Tissue engineering of functional trileaflet heart valves from human marrow stromal cells. Circulation 106:I143–I150
Schmidt D, Dijkman PE, Driessen-Mol A, Stenger R, Mariani C, Puolakka A, Rissanen M, Deichmann T, Odermatt B, Weber B, Emmert MY, Zund G, Baaijens FPT, Hoerstrup SP (2010) Minimally invasive implantation of living tissue engineered heart valves—a comprehensive approach from autologous vascular cells to stem cells. J Am Col Cardiol 3(56):510–520
Tanaka KA, Key NS, Levy JH (2009) Blood coagulation: hemostasis and thrombin regulation. Anesth Analg 108:1433–1446
Asahara T, Murohara T, Sullivan A et al (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964–967
Kim S, von Recum HA (2008) Endothelial stem cells and precursors for tissue engineering: cell source, differentiation, selection, and application. Tissue Eng B Rev 14:133–147
Kim S, von Recum HA (2009) Endothelial progenitor populations in differentiating embryonic stem cells I: identification and differentiation kinetics. Tissue Eng A 15:3709–3718
Hoerstrup SP, Kadner A, Breymann CI et al (2002) Living, autologous pulmonary artery conduits tissue engineered from human umbilical cord cells. Ann Thorac Surg 74:46–52
Kadner A, Hoerstrup SP, Tracy J et al (2002) Human umbilical cord cells: a new cell source for cardiovascular tissue engineering. Ann Thorac Surg 74:S1422–S1428
Kadner A, Zund G, Maurus C et al (2004) Human umbilical cord cells for cardiovascular tissue engineering: a comparative study. Eur J Cardiothorac Surg 25:635–641
Sipehia R, Martucci G, Lipscombe J (1996) Transplantation of human endothelial cell monolayer on artificial vascular prosthesis: the effect of growth-support surface chemistry, cell seeding density, ECM protein coating, and growth factors. Artif Cells Blood Substit Immobil Biotechnol 24:51–63
Schmidt D, Breymann C, Weber A et al (2004) Umbilical cord blood derived endothelial progenitor cells for tissue engineering of vascular grafts. Ann Thorac Surg 78:2094–2098
Schmidt D, Mol A, Neuenschwander S et al (2005) Living patches engineered from human umbilical cord derived fibroblasts and endothelial progenitor cells. Eur J Cardiothorac Surg 27:795–800
Schmidt D, Asmis LM, Odermatt B (2006) Engineered living blood vessels: functional endothelia generated from human umbilical cord-derived progenitors. Ann Thorac Surg 82:1465–1471
Schmidt D, Mol A, Odermatt B et al (2006) Engineering of biologically active living heart valve leaflets using human umbilical cord-derived progenitor cells. Tissue Eng 12:3223–3232
Breymann C, Schmidt D, Hoerstrup SP (2006) Umbilical cord cells as a source of cardiovascular tissue engineering. Stem Cell Rev 2:87–92
Parolini O, Soncini M, Evangelista M, Schmidt D (2009) Amniotic membrane and amniotic fluid-derived cells: potential tools for regenerative medicine? Regen Med 4:275–291
Pansky A, Roitzheim B, Tobiasch E (2007) Differentiation potential of adult human mesenchymal stem cells. Clin Lab 53:81–84
Tuan RS, Boland G, Tuli R (2003) Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther 5:32–45
Cao Y, Sun Z, Liao L et al (2005) Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun 332:370–379
DiMuzio P, Tulenko T (2007) Tissue engineering applications to vascular bypass graft development: the use of adipose-derived stem cells. J Vasc Surg 45:A99–A103
Breuer CK, Mettler BA, Anthony T et al (2004) Application of tissue-engineering principles to-ward the development of a semilunar heart valve substitute. Tissue Eng 10:1725–1736
Lichtenberg A, Cebotari S, Tudorache I et al (2007) Biological scaffolds for heart valve tissue engineering. Methods Mol Med 140:309–317
Sales VL, Engelmayr GC Jr, Johnson JA Jr et al (2007) Protein precoating of elastomeric tissue-engineering scaffolds increased cellularity, enhanced extracellular matrix protein production, and differentially regulated the phenotypes of circulating endothelial progenitor cells. Circulation 116:I55–I63
Affonso da Costa FD, Dohmen PM, Lopes SV et al (2004) Comparison of cryopreserved homografts and decellularized porcine heterografts implanted in sheep. Artif Organs 28:366–370
Allen BS, El-Zein C, Cuneo B et al (2002) Pericardial tissue valves and Gore-Tex conduits as an alternative for right ventricular outflow tract replacement in children. Ann Thorac Surg 74:771–777
Bielefeld MR, Bishop DA, Campbell DN et al (2001) Reoperative homograft right ventricular outflow tract reconstruction. Ann Thorac Surg 71:482–487
Carr-White GS, Glennan S, Edwards S et al (1999) Pulmonary autograft versus aortic homograft for rereplacement of the aortic valve: results from a subset of a prospective randomized trial. Circulation 100:II103–II106
Grauss RW, Hazekamp MG, van Vliet S et al (2003) Decellularization of rat aortic valve allografts reduces leaflet destruction and extracellular matrix remodeling. J Thorac Cardiovasc Surg 126:2003–2010
Stamm C, Khosravi A, Grabow N et al (2004) Biomatrix/polymer composite material for heart valve tissue engineering. Ann Thorac Surg 78:2084–2092
Hong H, Dong GN, Shi WJ et al (2008) Fabrication of biomatrix/polymer hybrid scaffold for heart valve tissue engineering in vitro. ASAIO J 54:627–632
Hong H, Dong N, Shi J et al (2009) Fabrication of a novel hybrid heart valve leaflet for tissue engineering: an in vitro study. Artif Organs 33:554–558
Neidert MR, Tranquillo RT (2006) Tissue-engineered valves with commissural alignment. Tissue Eng 12:891–903
Williams C, Johnson SL, Robinson PS et al (2006) Cell sourcing and culture conditions for fibrin-based valve constructs. Tissue Eng 12:1489–1502
Robinson PS, Johnson SL, Evans MC et al (2008) Functional tissue-engineered valves from cell-remodeled fibrin with commissural alignment of cell-produced collagen. Tissue Eng A 14:83–95
Syedain ZH, Weinberg JS, Tranquillo RT (2008) Cyclic distension of fibrin-based tissue constructs: evidence of adaptation during growth of engineered connective tissue. Proc Natl Acad Sci 6(105):6537–6542
Agrawal CM, Ray RB (2001) Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J Biomed Mater Res 55:141–150
Hutmacher DW, Schantz T, Zein I et al (2001) Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J Biomed Mater Res 55:203–216
Kessler B, Witholt B (2001) Factors involved in the regulatory network of polyhydroxyalkanoate metabolism. J Biotechnol 86:97–104
Sodian R, Hoerstrup SP, Sperling JS et al (2000) Evaluation of biodegradable, three-dimensional matrices for tissue engineering of heart valves. ASAIO J 46:107–110
Schaefermeier PK, Szymanski D, Weiss F et al (2009) Design and fabrication of three-dimensional scaffolds for tissue engineering of human heart valves. Eur Surg Res 42:49–53
Rabkin E, Hoerstrup SP, Aikawa M et al (2002) Evolution of cell phenotype and extracellular matrix in tissue-engineered heart valves during in-vitro maturation and in-vivo remodeling. J Heart Valve Dis 11:308–314
Mol A, Rutten MC, Driessen NJ et al (2006) Autologous human tissue-engineered heart valves: prospects for systemic application. Circulation 114:I152–I158
Kasimir MT, Rieder E, Seebacher G et al (2003) Comparison of different decellularization procedures of porcine heart valves. Int J Artif Organs 26:421–427
Steinhoff G, Stock U, Karim N et al (2000) Tissue engineering of pulmonary heart valves on allogenic acellular matrix conduits: in vivo restoration of valve tissue. Circulation 7(102):50–55
Leyh RG, Wilhelmi M, Rebe P et al (2003) In vivo repopulation of xenogeneic and allogeneic acellular valve matrix conduits in the pulmonary circulation. Ann Thorac Surg 75:1457–1463
Metzner A, Stock UA, Iino K, Fischer G, Huemme T, Boldt J, Braesen JH, Bein B, Renner J, Cremer J, Lutter G (2010) Percutaneous pulmonary valve replacement: autologous tissue-engineered valved stents. Cardiovasc Res 88:453–461
Curtil A, Pegg DE, Wilson A (1997) Repopulation of freeze-dried porcine valves with human fibroblasts and endothelial cells. J Heart Valve Dis 6:296–306
Wilson GJ, Courtman DW, Klement P et al (1995) Acellular matrix: a biomaterials approach for coronary artery bypass and heart valve replacement. Ann Thorac Surg 60:353–358
Bertiplaglia B, Ortolani F, Petrelli L et al (2003) Cell characterization of porcine aortic valve and decellularized leaflets repopulated with aortic valve interstitial cells: the VESALIO project. Ann Thorac Surg 75:1274–1282
Zeltinger J, Landeen LK, Alexander HG et al (2001) Development and characterization of tissue-engineered aortic valves. Tissue Eng 7:9–22
Rieder E, Kasimir MT, Silberhumer G et al (2004) Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to re-cellularization with human vascular cells. J Thorac Cardiovasc Surg 127:399–405
Tudorache I, Cebotari S, Sturz G et al (2007) Tissue engineering of heart valves: biomechanical and morphological properties of decellularized heart valves. J Heart Valve Dis 16:567–573
Booth C, Korossis SA, Wilcox HE et al (2002) Tissue engineering of cardiac valve prostheses I: development and histological characterization of an acellular porcine scaffold. J Heart Valve Dis 11:457–462
Knight RL, Booth C, Wilcox HE et al (2005) Tissue engineering of cardiac valves: re-seeding of acellular porcine aortic valve matrices with human mesenchymal progenitor cells. J Heart Valve Dis 14:806–813
Kim SS, Lim SH, Hong YS et al (2006) Tissue engineering of heart valves in vivo using bone marrow-derived cells. Artif Organs 30:554–557
Simon P, Kasimir MT, Seebacher G, Weigel G, Ullrich R, Salzer-Muhar U, Rieder E, Wolner E (2003) Early failure of the tissue engineered porcine heart valve SYNERGRAFT in pediatric patients. Eur J Cardiothorac Surg 23:1002–1006
Dohmen PM, Konertz W (2009) Tissue-engineered heart valve scaffolds. Ann Thorac Cardiovasc Surg 15:362–367
Takeuchi Y (2000) Risk of zoonosis in xenotransplantation. Transplant Proc 32:2698–2700
Weiss RA, Magre S, Takeuchi Y (2000) Infection hazards of xenotransplantation. J Infect 40:21–25
Mendelson K, Aikawa E, Mettler BA, Sales V, Martin D, Mayer JE, Schoen FJ (2007) Healing and remodeling of bioengineered pulmonary artery patches implanted in sheep. Cardiovasc Pathol 16(5):277–282
Burn TC, Petrovick MS, Hohaus S, Rollins BJ, Tenen DG (1994) Monocyte chemoattractant protein-1 gene is expressed in activated neutrophils and retinoic acid-induced human myeloid cell lines. Blood 15(84):2776–2783
Hoerstrup SP, Cummings Mrcs I, Lachat M et al (2006) Functional growth in tissue-engineered living, vascular grafts: follow-up at 100 weeks in a large animal model. Circulation 4(114):59–66
Moldovan NI, Goldschmidt-Clermont PJ, Parker-Thornburg J, Shapiro SD, Kolattukudy PE (2000) Contribution of monocytes/macrophages to compensatory neovascularization: the drilling of metalloelastase-positive tunnels in ischemic myocardium. Circ Res 87:378–384
Rehman J, Li J, Orschell CM, March KL (2003) Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 107:1164–1169
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8:958–969
Martinez FO, Gordon S, Locati M, Mantovani A (2006) Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 177:7303–7311
Arras M et al (1998) Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest 101:40–50
Heil M et al (2002) Blood monocyte concentration is critical for enhancement of collateral artery growth. Am J Physiol Heart Circ Physiol 283:2411–2419
Bergmann CE et al (2006) Arteriogenesis depends on circulating monocytes and macrophage accumulation and is severely depressed in op/op mice. J Leukoc Biol 80:59–65
Grunewald M et al (2006) VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell 124:175–189
Alon T et al (1995) Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med 1:1024–1028
Asahara T et al (1999) Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85:221–228
Roh JD, Sawh-Martinez R, Brennan MP, Jay SM, Devine L, Rao DA, Yi T, Mirensky TL, Nalbandian A, Udelsman B, Hibino N, Shinoka T, Saltzman WM, Snyder E, Kyriakides TR, Pober JS, Breuer CK (2010) Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci USA 9(107):4669–4674
Piemonti L, Guidotti LG, Battaglia M (2010) Modulation of early inflammatory reactions to promote engraftment and function of transplanted pancreatic islets in autoimmune diabetes. Adv Exp Med Biol 654:725–747
Acknowledgments
This work was supported by grants from the Swiss National Science Foundation [32-122273], Swiss South African Joint Research Programme of the State Secretariat for Education and Research, Swiss Government [EX25-2010] as well as the 7th Framework Programme, Life Valve, European Commission [242008].
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published as part of the Special Issue on “Implant devices: Biocompatibility, Tissue Engineering and Infection”.
Rights and permissions
About this article
Cite this article
Weber, B., Emmert, M.Y., Schoenauer, R. et al. Tissue engineering on matrix: future of autologous tissue replacement. Semin Immunopathol 33, 307–315 (2011). https://doi.org/10.1007/s00281-011-0258-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-011-0258-8