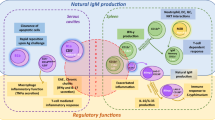
Abstract
Numerous studies in several species have shown that certain subsets of T and B lymphocytes express antigen receptors which are either semi-invariant, or germline encoded, and often autoreactive. In the case of B cells they appear to use a distinct immune recognition strategy during developmental selection and functional activation. These B cells respond to foreign antigens, and have the ability to protect against a variety of infections; however, they can also react with self or neoself antigens. They appear to use the latter as positively selecting ligands facilitating their entry into and maintenance in a functional repertoire, as well as providing cues for positioning themselves in strategic microenvironmental niches in the immune system and at interfaces with the environment. These innate-like B cell subsets form a bridge between the rapidly occurring innate immune responses, and the slower acting primary, T cell-dependent, adaptive antibody response by providing a rapid T cell-independent antibody response.

Similar content being viewed by others
References
Balazs M, Martin F, Zhou T, et al (2002) Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity 17:341
Benca R, Quintans J, Kearney JF, et al (1980) Studies on phosphorylcholine-specific T cell idiotypes and idiotype-specific immunity. Mol Immunol 17:823
Bendelac A, Bonneville M, Kearney JF (2001) Autoreactivity by design: innate B and T lymphocytes. Nat Rev Immunol 1:177
Benedict CL, Kearney JF (1999) Increased junctional diversity in fetal B cells results in a loss of protective anti-phosphorylcholine antibodies in adult mice. Immunity 10:607
Binder CJ, Hartvigsen K, Chang MK, et al (2004) IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest 114:427
Briles DE, Nahm M, Schroer K, et al (1981) Anti-phosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J Exp Med 153:694
Cancro MP, Kearney JF (2004) B cell positive selection: road map to the primary repertoire? J Immunol 173:15
Chen X, Martin F, Forbush KA, et al (1997) Evidence for selection of a population of multi-reactive B cells into the splenic marginal zone. Int Immunol 9:27
Etlinger HM, Heusser CH (1986) T15 dominance in BALB/c mice is not controlled by environmental factors. J Immunol 136:1988
Fagarasan S, Honjo T (2004) Regulation of IgA synthesis at mucosal surfaces. Curr Opin Immunol 16:277
Fagarasan S, Kinoshita K, Muramatsu M, et al (2001) In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature 413:639
Feeney AJ (1991) Junctional sequences of fetal T cell receptor beta chains have few N regions. J Exp Med 174:115
Feeney AJ (1992) Comparison of junctional diversity in the neonatal and adult immunoglobulin repertoires. Int Rev Immunol 8:113
Girardi M, Lewis J, Glusac E, et al (2002) Resident skin-specific γδ T cells provide local, nonredundant regulation of cutaneous inflammation. J Exp Med 195:855
Greenspan NS, Fulton RJ, Davie JM (1986) Analysis of anti-streptococcal group A carbohydrate idiotope levels in sera: correlation of magnitude of expression with idiotope position and VK haplotype. J Immunol 137:228
Gu H, Tarlinton D, Muller W, et al (1991) Most peripheral B cells in mice are ligand selected. J Exp Med 173:1357
Hardy RR, Carmack CE, Shinton SA, et al (1989) A single VH gene is utilized predominantly in anti-BrMRBC hybridomas derived from purified Ly-1 B cells. Definition of the VH11 family. J Immunol 142:3643
Hardy RR, Hayakawa K (1991) A developmental switch in B lymphopoiesis. Proc Natl Acad Sci USA 88:11550
Hayakawa K, Asano M, Shinton SA, et al (1999) Positive selection of natural autoreactive B cells. Science 285:113
Hayakawa K, Asano M, Shinton SA, et al (2003) Positive selection of anti-thy-1 autoreactive B-1 cells and natural serum autoantibody production independent from bone marrow B cell development. J Exp Med 197:87
Hayday A, Theodoridis E, Ramsburg E, et al (2001) Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat Immunol 2:997
Kearney JF, Barletta R, Quan ZS, et al (1981) Monoclonal vs. heterogeneous anti-H-8 antibodies in the analysis of the anti-phosphorylcholine response in BALB/c mice. Eur J Immunol 11:877
Kearney JF, McCarthy MTM, Stohrer R, et al (1985) Induction of germ-line anti-alpha 1–3 dextran antibody responses in mice by members of the Enterobacteriaceae family. J Immunol 135:3468
Lopes-Carvalho T, Kearney JF (2004) Development and selection of marginal zone B cells. Immunol Rev 197:192
Martin F, Kearney JF (2000) B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory”. Immunol Rev 175:70
Martin F, Kearney JF (2000) Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity 12:39
Mercolino TJ, Arnold LW, Hawkins LA, et al (1988) Normal mouse peritoneum contains a large population of Ly-1+ (CD5) B cells that recognize phosphatidyl choline. Relationship to cells that secrete hemolytic antibody specific for autologous erythrocytes. J Exp Med 168:687
Ochsenbein AF, Fehr T, Lutz C, et al (1999) Control of early viral and bacterial distribution and disease by natural antibodies. Science 286:2156
Oliver AM, Martin F, Gartland GL, et al (1997) Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur J Immunol 27:2366
Oliver AM, Martin F, Kearney JF (1999) IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J Immunol 162:7198
Paciorkowski N, Port P, Schultz LD, et al (2000) B1 B lymphocytes play a critical role in host protection against lymphatic filarial parasites. J Exp Med 191:731
Shaw PX, Goodyear CS, Chang MK, et al (2003) The autoreactivity of anti-phosphorylcholine antibodies for atherosclerosis-associated neo-antigens and apoptotic cells. J Immunol 170:6151
Shaw PX, Horkko S, Chang MK, et al (2000) Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest 105:1731
Shikhman AR, Greenspan NS, Cunningham MW (1993) A subset of mouse monoclonal antibodies cross-reactive with cytoskeletal proteins and group A streptococcal M proteins recognizes N-acetyl-beta-d-glucosamine. J Immunol 151:3902
Sigal NH, Gearhart PJ, Klinman NR (1975) The frequency of phosphorylcholine-specific B cells in conventional and germfree BALB/C mice. J Immunol 114:1354
Sigal NH, Gearhart PJ, Press JL, et al. 1976) Late acquisition of a germ line antibody specificity. Nature 259:51
Turner JR, Tartakoff AM, Greenspan NS (1990) Cytologic assessment of nuclear and cytoplasmic O-linked N-acetylglucosamine distribution by using anti-streptococcal monoclonal antibodies. Proc Natl Acad Sci USA 87:5608
Vakil M, Kearney JF (1991) Functional relationship between T15 and J558 idiotypes in BALB/c mice. Dev Immunol 1:213
Wang H, Clarke SH (2004) Positive selection focuses the VH12 B-cell repertoire towards a single B1 specificity with survival function. Immunol Rev 197:51
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kearney, J.F. Innate-like B cells. Springer Semin Immun 26, 377–383 (2005). https://doi.org/10.1007/s00281-004-0184-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-004-0184-0