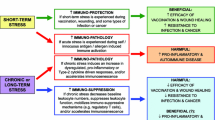
Abstract
It has long been known that immunization with a protein by itself is often not sufficient to stimulate immunity, and may instead induce tolerance. To elicit productive immune responses exogenous adjuvants need to be co-injected with an antigen. One important class of adjuvants are the unique (non-mammalian) components of microbes. It is now believed that an adjuvant is required for immunity because the immune system evolved to respond to dangerous situations such as infections, and the presence of an adjuvant is the mechanism used to identify these situations. However, there are some circumstances where immune responses are generated in the apparent absence of any microbial or other exogenous adjuvant. Such situations include immune responses to transplants, tumors, autoimmunity and possibly certain viral infections. It has been postulated that in these situations the danger signals come from endogenous adjuvants that are released from dying cells. There is abundant evidence that dead cells are immunogenic, and recently it has been shown that cells contain endogenous adjuvant activities that are released after death. Some actual and putative endogenous adjuvants, such as monosodium urate and heat shock proteins, have been identified and there are others whose identities are not yet known. The potential biological roles of this class of adjuvants are discussed.


Similar content being viewed by others
References
Albert ML, Sauter B, Bhardwaj N (1998) Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86
Alexopoulou L, Holt AC, Medzhitov R, et al (2001) Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732
Asea A, Kraeft SK, Kurt-Jones EA, et al (2000) HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med 6:435
Asea A, Rehli M, Kabingu E, et al (2002) Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem 277:15028
Baker-LePain JC, Sarzotti M, Fields TA, et al (2002) GRP94 (gp96) and GRP94 N-terminal geldanamycin binding domain elicit tissue nonrestricted tumor suppression. J Exp Med 196:1447
Barba D, Hardin J, Sadelain M, et al (1994) Development of anti-tumor immunity following thymidine kinase-mediated killing of experimental brain tumors. Proc Natl Acad Sci USA 91:4348
Barton GM, Medzhitov R (2003) Toll-like receptor signaling pathways. Science 300:1524
Basu S, Binder RJ, Suto R, et al (2000) Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol 12:1539
Bausinger H, Lipsker D, Ziylan U, et al (2002) Endotoxin-free heat-shock protein 70 fails to induce APC activation. Eur J Immunol 32:3708
Belvin MP, Anderson KV (1996) A conserved signaling pathway: the Drosophila toll-dorsal pathway. Annu Rev Cell Dev Biol 12:393
Bethke K, Staib F, Distler M, et al (2002) Different efficiency of heat shock proteins (HSP) to activate human monocytes and dendritic cells: superiority of HSP60. J Immunol 169:6141
Bevan MJ (1976) Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med 143:1283
Binder RJ, Anderson KM, Basu S, et al (2000) Cutting edge: heat shock protein gp96 induces maturation and migration of CD11c+ cells in vivo. J Immunol 165:6029
Bretscher P, Cohn M (1970) A theory of self-nonself discrimination. Science 169:1042
Buttiglieri S, Galetto A, Forno S, et al (2003) Influence of drug-induced apoptotic death on processing and presentation of tumor antigens by dendritic cells. Int J Cancer 106:516
Caruso M, Panis Y, Gagandeep S, et al (1993) Regression of established macroscopic liver metastases after in situ transduction of a suicide gene. Proc Natl Acad Sci USA 90:7024
Chang JW, Peng M, Vaquerano JE, et al (2000) Induction of Th1 response by dendritic cells pulsed with autologous melanoma apoptotic bodies. Anticancer Res 20:1329
Chen Z, Moyana T, Saxena A, et al (2001) Efficient antitumor immunity derived from maturation of dendritic cells that had phagocytosed apoptotic/necrotic tumor cells. Int J Cancer 93:539
Claman HN (1963) Tolerance to a protein antigen in adult mice and the effect of nonspecific factors. J Immunol 91:833
Consalvo M, Mullen CA, Modesti A, et al (1995) 5-Fluorocytosine-induced eradication of murine adenocarcinomas engineered to express the cytosine deaminase suicide gene requires host immune competence and leaves an efficient memory. J Immunol 154:5302
Dresser DW (1961) Effectiveness of lipid and lipidophilic substances as adjuvants. Nature 191:1169
Dresser DW (1962) Specific inhibition of antibody production. II. Paralysis induced in adult mice by small quantities of protein antigen. Immunology 5:378
Dresser DW (1968) An assay for adjuvanticity. Clin Exp Immunol 3:877
Feng H, Zeng Y, Graner MW, et al (2002) Stressed apoptotic tumor cells stimulate dendritic cells and induce specific cytotoxic T cells. Blood 100:4108
Feng H, Zeng Y, Whitesell L, et al (2001) Stressed apoptotic tumor cells express heat shock proteins and elicit tumor-specific immunity. Blood 97:3505
Ferguson TA, Herndon J, Elzey B, et al (2002) Uptake of apoptotic antigen-coupled cells by lymphoid dendritic cells and cross-priming of CD8(+) T cells produce active immune unresponsiveness. J Immunol 168:5589
Ferlazzo G, Semino C, Spaggiari GM, et al (2000) Dendritic cells efficiently cross-prime HLA class I-restricted cytolytic T lymphocytes when pulsed with both apoptotic and necrotic cells but not with soluble cell-derived lysates. Int Immunol 12:1741
Flohe SB, Bruggemann J, Lendemans S, et al (2003) Human heat shock protein 60 induces maturation of dendritic cells versus a Th1-promoting phenotype. J Immunol 170:2340
Foley EJ (1953) Antigenic properties of methylcholanthrene-induced tumors in mice of the strain of origin. Cancer Res 13:835
Freund J (1953) The response of immunized animals to specific and non-specific stimuli. Columbia University Press, New York
Gallucci S, Lolkema M, Matzinger P (1999) Natural adjuvants: endogenous activators of dendritic cells. Nat Med 5:1249
Gao B, Tsan MF (2003) Endotoxin contamination in recombinant human heat shock protein 70 (Hsp70) preparation is responsible for the induction of tumor necrosis factor alpha release by murine macrophages. J Biol Chem 278:174
Gao B, Tsan MF (2003) Recombinant human heat shock protein 60 does not induce the release of tumor necrosis factor alpha from murine macrophages. J Biol Chem 278:22523
Gery I, Gershon RK, Waksman BH (1972) Potentiation of the T-lymphocyte response to mitogens. I. The responding cell. J Exp Med 136:128
Gillis S, Mizel SB (1981) T-cell lymphoma model for the analysis of interleukin 1-mediated T-cell activation. Proc Natl Acad Sci USA 78:1133
Glenny AT, Buttle GAH, Stevens MF (1931) Rate of disappearance of diphtheria toxoid injected into rabbits and guinea-pigs: toxoid precipitated with alum. J Pathol Bacteriol 34:267
Glenny AT, Pope CG, Waddington H, et al (1926) XXIII-the antigenic value of toxiod precipitated by potassium alum. J Pathol Bacteriol 29:38
Goldszmid RS, Idoyaga J, Bravo AI, et al (2003) Dendritic cells charged with apoptotic tumor cells induce long-lived protective CD4+ and CD8+ T cell immunity against B16 melanoma. J Immunol 171:5940
Guillot L, Balloy V, McCormack FX, et al (2002) Cutting edge: the immunostimulatory activity of the lung surfactant protein-A involves Toll-like receptor 4. J Immunol 168:5989
Heath WR, Carbone FR (2001) Cross-presentation in viral immunity and self-tolerance. Nat Rev Immunol 1:126
Hoffmann TK, Meidenbauer N, Dworacki G, et al (2000) Generation of tumor-specific T-lymphocytes by cross-priming with human dendritic cells ingesting apoptotic tumor cells. Cancer Res 60:3542
Horwitz MS, Ilic A, Fine C, et al (2002) Presented antigen from damaged pancreatic beta cells activates autoreactive T cells in virus-mediated autoimmune diabetes. J Clin Invest 109:79
Huang AY, Golumbek P, Ahmadzadeh M, et al (1994) Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science 264:961
Ignatius R, Marovich M, Mehlhop E, et al (2000) Canarypox virus-induced maturation of dendritic cells is mediated by apoptotic cell death and tumor necrosis factor alpha secretion. J Virol 74:11329
Inaba K, Turley S, Yamaide F, et al (1998) Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J Exp Med 188:2163
Ishii S, Hiroishi K, Eguchi J, et al (2003) Dendritic cell maturation induced by delivery of ultraviolet-mediated apoptotic colorectal cancer cell lines. Anticancer Res 23:2457
Jaeschke H, Lemasters JJ (2003) Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology 125:1246
Janeway CA Jr (1989) Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol 54:1
Janeway CA Jr (1992) The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today 13:11
Jenkins MK, Schwartz RH (1987) Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med 165:302
Jenne L, Arrighi JF, Jonuleit H, et al (2000) Dendritic cells containing apoptotic melanoma cells prime human CD8+ T cells for efficient tumor cell lysis. Cancer Res 60:4446
June CH, Bluestone JA, Nadler LM, et al (1994) The B7 and CD28 receptor families. Immunol Today 15:321
Kaisho T, Akira S (2002) Toll-like receptors as adjuvant receptors. Biochim Biophys Acta 1589:1
Kaye J, Gillis S, Mizel SB, et al (1984) Growth of a cloned helper T cell line induced by a monoclonal antibody specific for the antigen receptor: interleukin 1 is required for the expression of receptors for interleukin 2. J Immunol 133:1339
Kim KW, Kim SH, Shin JG, et al (2004) Direct injection of immature dendritic cells into irradiated tumor induces efficient antitumor immunity. Int J Cancer 109:685
Kol A, Bourcier T, Lichtman AH, et al (1999) Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J Clin Invest 103:571
Kol A, Lichtman AH, Finberg RW, et al (2000) Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J Immunol 164:13
Kotera Y, Shimizu K, Mule JJ (2001) Comparative analysis of necrotic and apoptotic tumor cells as a source of antigen(s) in dendritic cell-based immunization. Cancer Res 61:8105
Kovacsovics-Bankowski M, Clark K, Benacerraf B, et al (1993) Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc Natl Acad Sci USA 90:4942
Kuppner MC, Gastpar R, Gelwer S, et al (2001) The role of heat shock protein (hsp70) in dendritic cell maturation: hsp70 induces the maturation of immature dendritic cells but reduces DC differentiation from monocyte precursors. Eur J Immunol 31:1602
Kurt-Jones EA, Chan M, Zhou S, et al (2004) Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci USA 101:1315
Kurt-Jones EA, Popova L, Kwinn L, et al (2000) Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol 1:398
Kurts C, Miller JF, Subramaniam RM, et al (1998) Major histocompatibility complex class I-restricted cross-presentation is biased towards high dose antigens and those released during cellular destruction. J Exp Med 188:409
Lafferty KJ, Cunningham AJ (1975) A new analysis of allogeneic interactions. Aust J Exp Biol Med Sci 53:27
Lund JM, Alexopoulou L, Sato A, et al (2004) Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci USA 101:5598
Majno G, Joris I (1995) Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol 146:3
Mastro AM, Mueller GC (1974) Synergistic action of phorbol esters in mitogen-activated bovine lymphocytes. Exp Cell Res 88:40
Matzinger P (1994) Tolerance, danger, and the extended family. Annu Rev Immunol 12:991
Matzinger P (1998) An innate sense of danger. Semin Immunol 10:399
Medzhitov R, Preston-Hurlburt P, Janeway CA Jr (1997) A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394
Melcher A, Todryk S, Hardwick N, et al (1998) Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nat Med 4:581
Millar DG, Garza KM, Odermatt B, et al (2003) Hsp70 promotes antigen-presenting cell function and converts T-cell tolerance to autoimmunity in vivo. Nat Med 9:1469
Morelli AE, Larregina AT, Shufesky WJ, et al (2003) Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: dependence on complement receptors and effect on cytokine production. Blood 101:611
Mullen CA, Coale MM, Lowe R, et al (1994) Tumors expressing the cytosine deaminase suicide gene can be eliminated in vivo with 5-fluorocytosine and induce protective immunity to wild type tumor. Cancer Res 54:1503
Nowak AK, Lake RA, Marzo AL, et al (2003) Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol 170:4905
Ohashi K, Burkart V, Flohe S, et al (2000) Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol 164:558
Okamura Y, Watari M, Jerud ES, et al (2001) The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem 276:10229
Pietra G, Mortarini R, Parmiani G, et al (2001) Phases of apoptosis of melanoma cells, but not of normal melanocytes, differently affect maturation of myeloid dendritic cells. Cancer Res 61:8218
Prehn RT, Main JM (1957) Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst 18:769
Quill H, Schwartz RH (1987) Stimulation of normal inducer T cell clones with antigen presented by purified Ia molecules in planar lipid membranes: specific induction of a long-lived state of proliferative nonresponsiveness. J Immunol 138:3704
Rad AN, Pollara G, Sohaib SM, et al (2003) The differential influence of allogeneic tumor cell death via DNA damage on dendritic cell maturation and antigen presentation. Cancer Res 63:5143
Raman K, Mohan C (2003) Genetic underpinnings of autoimmunity—lessons from studies in arthritis, diabetes, lupus and multiple sclerosis. Curr Opin Immunol 15:651
Ramon G (1925) Sur l’augmentation anormale de l’antitoxine chez les chevaux producteurs de serum antidiptherique. Bull Soc Centr Med Vet 101:227
Ronchetti A, Rovere P, Iezzi G, et al (1999) Immunogenicity of apoptotic cells in vivo: role of antigen load, antigen-presenting cells, and cytokines. J Immunol 163:130
Rosenstreich DL, Mizel SB (1979) Signal requirements for T lymphocyte activation. I. Replacement of macrophage function with phorbol myristic acetate. J Immunol 123:1749
Rovere P, Sabbadini MG, Vallinoto C, et al (1999) Delayed clearance of apoptotic lymphoma cells allows cross-presentation of intracellular antigens by mature dendritic cells. J Leukoc Biol 66:345
Rovere P, Vallinoto C, Bondanza A, et al (1998) Bystander apoptosis triggers dendritic cell maturation and antigen-presenting function. J Immunol 161:4467
Russo V, Tanzarella S, Dalerba P, et al (2000) Dendritic cells acquire the MAGE-3 human tumor antigen from apoptotic cells and induce a class I-restricted T cell response. Proc Natl Acad Sci USA 97:2185
Sauter B, Albert ML, Francisco L, et al (2000) Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med 191:423
Savill J, Dransfield I, Gregory C, et al (2002) A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol 2:965
Scheffer SR, Nave H, Korangy F, et al (2003) Apoptotic, but not necrotic, tumor cell vaccines induce a potent immune response in vivo. Int J Cancer 103:205
Schnurr M, Scholz C, Rothenfusser S, et al (2002) Apoptotic pancreatic tumor cells are superior to cell lysates in promoting cross-priming of cytotoxic T cells and activate NK and gammadelta T cells. Cancer Res 62:2347
Shaif-Muthana M, McIntyre C, Sisley K, et al (2000) Dead or alive: immunogenicity of human melanoma cells when presented by dendritic cells. Cancer Res 60:6441
Sharpe AH, Freeman GJ (2002) The B7-CD28 superfamily. Nat Rev Immunol 2:116
Shi Y, Evans JE, Rock KL (2003) Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425:516
Shi Y, Rock KL (2002) Cell death releases endogenous adjuvants that selectively enhance immune surveillance of particulate antigens. Eur J Immunol 32:155
Shi Y, Zheng W, Rock KL (2000) Cell injury releases endogenous adjuvants that stimulate cytotoxic T cell responses. Proc Natl Acad Sci USA 97:14590
Sigal LJ, Crotty S, Andino R, et al (1999) Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature 398:77
Singh-Jasuja H, Hilf N, Scherer HU, et al (2000) The heat shock protein gp96: a receptor-targeted cross-priming carrier and activator of dendritic cells. Cell Stress Chaperones 5:462
Singh-Jasuja H, Scherer HU, Hilf N, et al (2000) The heat shock protein gp96 induces maturation of dendritic cells and down-regulation of its receptor. Eur J Immunol 30:2211
Smiley ST, King JA, Hancock WW (2001) Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol 167:2887
Somersan S, Larsson M, Fonteneau JF, et al (2001) Primary tumor tissue lysates are enriched in heat shock proteins and induce the maturation of human dendritic cells. J Immunol 167:4844
Spisek R, Chevallier P, Morineau N, et al (2002) Induction of leukemia-specific cytotoxic response by cross-presentation of late-apoptotic leukemic blasts by autologous dendritic cells of nonleukemic origin. Cancer Res 62:2861
Srivastava PK, Maki RG (1991) Stress-induced proteins in immune response to cancer. Curr Top Microbiol Immunol 167:109
Steinman RM, Turley S, Mellman I, et al (2000) The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med 191:411
Strome SE, Voss S, Wilcox R, et al (2002) Strategies for antigen loading of dendritic cells to enhance the antitumor immune response. Cancer Res 62:1884
Stuart LM, Lucas M, Simpson C, et al (2002) Inhibitory effects of apoptotic cell ingestion upon endotoxin-driven myeloid dendritic cell maturation. J Immunol 168:1627
Subklewe M, Paludan C, Tsang ML, et al (2001) Dendritic cells cross-present latency gene products from Epstein-Barr virus-transformed B cells and expand tumor-reactive CD8(+) killer T cells. J Exp Med 193:405
Tabeta K, Georgel P, Janssen E, et al (2004) Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci USA 101:3516
Takeda K, Kaisho T, Akira S (2003) Toll-like receptors. Annu Rev Immunol 21:335
Termeer C, Benedix F, Sleeman J, et al (2002) Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med 195:99
Turley S, Poirot L, Hattori M, et al (2003) Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J Exp Med 198:1527
Udono H, Srivastava PK (1993) Heat shock protein 70-associated peptides elicit specific cancer immunity. J Exp Med 178:1391
Vabulas RM, Ahmad-Nejad P, da Costa C, et al (2001) Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem. 276:31332
Vabulas RM, Ahmad-Nejad P, Ghose S, et al (2002) HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem 277:15107
Vabulas RM, Braedel S, Hilf N, et al (2002) The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J Biol Chem 277:20847
Wan T, Zhou X, Chen G, et al (2004) Novel heat shock protein Hsp70L1 activates dendritic cells and acts as a Th1 polarizing adjuvant. Blood 103:1747
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rock, K.L., Hearn, A., Chen, CJ. et al. Natural endogenous adjuvants. Springer Semin Immun 26, 231–246 (2005). https://doi.org/10.1007/s00281-004-0173-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-004-0173-3