Abstract.
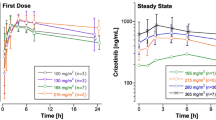
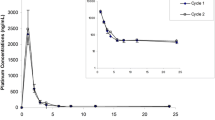
Purpose: To compare etoposide pharmacokinetics following administration of high-dose etoposide and etoposide phosphate, a water-soluble prodrug of etoposide. Bioequivalence was assessed using a two-treatment randomized crossover design. Methods: Ten patients with high-risk or relapsed lymphoma were treated with a sequential high-dose chemotherapy. They were randomized to receive either 3×400 mg/m2 etoposide or an equimolar amount of etoposide phosphate (as 1-h infusions on three consecutive days) in the first course and the alternative drug in the second course. Serial plasma and ultrafiltered plasma samples were collected and analysed for etoposide by a reversed-phase HPLC method with UV and electrochemical detection. Pharmacokinetic parameters were estimated using a two-compartment model. Bioequivalence was assessed calculating the 90% confidence intervals (CI) for the ratios of the geometric means of AUC0-∞ and additionally of Cmax of etoposide derived from etoposide phosphate relative to etoposide in plasma and ultrafiltered plasma as point estimates (level of significance α<0.05). Results: Pharmacokinetic parameters of etoposide were comparable in both treatment arms except that terminal half-life was significantly shorter and apparent Vss in ultrafiltered plasma was significantly larger following administration of the prodrug. The point estimates for AUC0-∞ of etoposide derived from etoposide phosphate relative to etoposide were 102.9% and 88.4% for plasma and ultrafiltered plasma, respectively. The 90% CIs were in the range from 80% to 125% where bioequivalence can be assumed. The point estimates of Cmax on day 3 of chemotherapy were 96.5% and 81.7% in plasma and ultrafiltrate with the 90% CI in ultrafiltered plasma being out of the range from 80% to 125%. Conclusion: With respect to total drug exposure, represented by AUC0-∞, high-dose etoposide phosphate is bioequivalent to high-dose etoposide.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Reif, S., Kingreen, D., Kloft, C. et al. Bioequivalence investigation of high-dose etoposide and etoposide phosphate in lymphoma patients. Cancer Chemother Pharmacol 48, 134–140 (2001). https://doi.org/10.1007/s002800100280
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s002800100280