Abstract
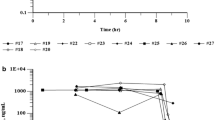
A total of 14 Chinese patients with inoperable hepatocellular carcinoma received a liposomal formulation of daunorubicin (DaunoXome) at a dose equivalent to 100 mg/m2 of the free drug every 3 weeks. Altogether, 12 patients were assessable for response; 2 patients had stable disease for 8 weeks, but all eventually developed progressive disease and there was no responder. The drug was well tolerated, with no evidence of cardiac toxicity being observed. Deterioration of liver-function tests was attributed to progressive tumors in the terminal stage of the disease. Pharmacokinetics studies revealed a biexponential decay for daunorubicin in association with mean initial and terminal half-lives of 1.8 and 7.4 h, respectively, and a mean total clearance of 15.0 ± 5.5 ml/min. The AUC ratio between the metabolite daunorubicinol and daunorubicin was 0.07. These data differ markedly from the pharmacokinetics of the free drug.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 13 August 1998 / Accepted: 13 January 1999
Rights and permissions
About this article
Cite this article
Yeo, W., Chan, K., Mukwaya, G. et al. Phase II studies with DaunoXome in patients with nonresectable hepatocellular carcinoma: clinical and pharmacokinetic outcomes. Cancer Chemother Pharmacol 44, 124–130 (1999). https://doi.org/10.1007/s002800050956
Issue Date:
DOI: https://doi.org/10.1007/s002800050956