Abstract
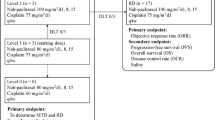
As a dose-response relationship has been suggested for cisplatin, it appeared attractive to explore high-dose-intensity regimens in non-small-cell lung cancer. In a phase I study of weekly administration of cisplatin combined with oral etoposide we achieved a cisplatin dose intensity of 52.5 – 60 mg/m2 per week in most patients. We subsequently explored this regimen in advanced non-small-cell lung cancer. Patients were treated with cisplatin infused at 70 mg/m2 on days 1, 8, 15 and 29, 36, 43 in combination with oral etoposide given at 50 mg on days 1 – 15 and 29 – 43. Patients showing stable disease or a better response were continued on treatment with oral etoposide given at 50 mg/m2 per day on days 1 – 21 every 28 days for a maximum of four cycles. In all, 22 patients with stage III disease and 31 patients with stage IV disease entered the study. The median number of cisplatin administration was 6 per patient; 17 patients reached the planned cisplatin dose intensity of 60 mg/m2 per week, 11 patients achieved 52.5 mg/m2 per week, and 7 patients reached 47 mg/m2 per week. Overall, 11 of 21 stage III patients had a partial response [response rate 51%, 95% confidence interval (CI) 36 – 81%], as did 9 of 28 patients with stage IV disease (32%; 95% CI 15 – 49%). Toxicity was mainly hematologic, with leukocytopenia being the most frequent cause of treatment delay. Nephrotoxicity of grade 1 was observed in seven patients. Two patients developed clinical hearing loss. With this schedule a high median cisplatin dose intensity of 52.5 – 60 mg/m2 per week was reached. The 51% response rate achieved in stage III disease makes this schedule attractive for further exploration; however, it is not recommended for routine use in stage IV disease.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 25 August 1996 / Accepted: 20 January 1997
Rights and permissions
About this article
Cite this article
Planting, A., Kho, S., van der Burg, M. et al. A phase II study of weekly high-dose cisplatin combined with oral etoposide in advanced non-small-cell lung cancer. Cancer Chemother Pharmacol 40, 347–352 (1997). https://doi.org/10.1007/s002800050668
Issue Date:
DOI: https://doi.org/10.1007/s002800050668