Abstract
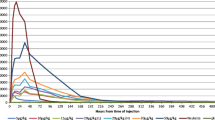
Recent studies in human bone-marrow culture and healthy human volunteers suggest that lenograstim [glycosylated, recombinant human granulocyte colony-stimulating factor (rHuG-CSF) produced in Chinese hamster ovary (CHO) cells] has greater in vivo potency than filgrastim [nonglycosylated, methionine-extended recombinant human granulocyte colony-stimulating factor (r-metHuG-CSF) produced in Escherichia coli]. To confirm and extend these results we investigated thein vivopotency of both products in normal rats and neutropenic CD rats as an animal model of chemotherapy-induced neutropenia. In normal rats, groups of eight normal male CD rats received four subcutaneous doses of 10, 30, or 100 μg/kg filgrastim or lenograstim on days 1 – 4 of the study, whereas a control group received the vehicle. Blood samples were collected from each animal before treatment (day – 5) and on days 2, 3, 5, 8, and 12 of the study for determination of red blood cell (RBC), platelet, white blood cell (WBC), and differential counts. rHuG-CSF and r-metHuG-CSF produced increased WBC counts, principally due to elevated absolute neutrophil counts (ANCs); on days 2, 3, and 5, all groups receiving rG-CSF had ANCs that increased in a progressive and dose-related manner. With the exception of a single value, mean ANCs obtained on days 2, 3, and 5 in lenograstim-treated groups were higher (statistically significant on day 3 at 30 and 100 μg/kg and on day 5 at 10, 30, and 100 μg/kg) than the respective values obtained in filgrastim-treated groups. No compound-related effect was noted in RBC or platelet parameters. Neutropenia was induced in male CD rats (12 animals/group) with a single intraperitoneal dose of 50 mg/kg cyclophosphamide (CPA) on day 0. On days 1 – 4, CPA-treated groups were treated with the vehicle (control) or with filgrastim or lenograstim at 30 or 100 μg/kg per day. An additional group was not treated with CPA and served as the absolute control group. Blood was collected from alternating subgroups on study day – 5 (pretest) and on days 2, 3, 4, 5, 6, 8, 9, and 12 for determination of RBC, platelet, WBC, and differential counts. No major adverse in-life effect was noted in neutropenic rats. Maximal depression of WBCs and ANCs occurred on day 5, followed by recovery to normal values by days 9 (ANC) and 12 (WBC). On day 3 and days 5 – 9, rHuG-CSF- and metHuG-CSF-treated groups had marked and dose-related increases in WBCs as compared with CPA-treated controls, principally due to elevated ANCs. With the exception of a few values, mean ANC values obtained in lenograstim-treated groups were consistently higher than the respective values obtained in filgrastim-treated groups; the difference was statistically significant on day 3 (30-μg/kg groups) and on days 6 and 8 (100-μg/kg groups). In conclusion, treatment of normal and neutropenic CD rats with lenograstim resulted in a dose-related elevation of ANCs that was consistently and significantly higher than the response to identical doses of filgrastim. These results suggest that lenograstim, the glycosylated form of rG-CSF, has superior in vivo potency in normal and neutropenic animals as compared with filgrastim, the nonglycosylated form of rG-CSF.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 4 April 1996 / Accepted: 24 June 1996
Rights and permissions
About this article
Cite this article
Nohynek, G., Plard, J., Wells, M. et al. Comparison of the potency of glycosylated and nonglycosylated recombinant human granulocyte colony-stimulating factors in neutropenic and nonneutropenic CD rats. Cancer Chemother Pharmacol 39, 259–266 (1996). https://doi.org/10.1007/s002800050570
Issue Date:
DOI: https://doi.org/10.1007/s002800050570