Abstract
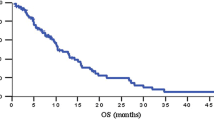
Gemcitabine is a fluorine-substituted cytarabine analog with broad experimental antitumor activity. It’s activity was explored in chemotherapy-naive patients with advanced progressive renal-cell carcinoma. A total of 39 patients were included in the study, of whom 37 were fully evaluable. In five patients the primary tumor remained in situ. Gemcitabine at 800 mg/m2 was given as a weekly 30-min infusion for 3 consecutive weeks followed by 1 week of rest. One complete response and two partial responses were observed giving a response rate of 8.1% [95% confidence interval (CI), 2–22%). The duration of the responses is currently 32, 15, and 19 months, respectively. The median survival for all patients was 12.3 months. Gemcitabine was generally well tolerated, with nausea and vomiting (20.5% grade III) and neutropenia (5.3% grade III) being the most significant side effects. Gemcitabine given at this dose level and on this schedule has only limited activity in advanced renal-cell carcinoma.
Similar content being viewed by others
References
Ritchie AWS, Chisholm GD (1983) The natural history of renal cell carcinoma. Semin Oncol 10: 390–400
De Forges A, Rey A, Klink M, et al (1988) Prognostic factors in adult metastatic renal carcinoma: a multivariate analysis. Semin Surg Oncol 4:149–154
Montie JE, Stewart BH, Straffon RA, et al (1977) The role of adjunctive nephrectomy in patients with metastatic renal cell carcinoma. J Urol 117:272–275
Harris DT (1983) Hormonal therapy and chemotherapy of renal cell carcinoma. Semin Oncol 10:422–430
Yagoda A, Bander NH (1989) Failure of cytotoxic chemotherapy, 1983-1988, and the emerging role of monoclonal antibodies for renal cancer. Urol Int 44:338–345
McCune CS (1983) Immunologic therapies in kidney carcinoma. Semin Oncol 10:431–436
Rosenberg SA, Yang JC, Topalian SL, et al (1994) Treatment of 238 consecutive patients with metatastatic melanoma or renal cell cancer using high-dose bolus interleukin-2. JAMA 271:907–913
Pollera CF, Ceribelli A, Grecco M, et al (1992) Weekly gemcitabine: a phase I study with short and prolonged infusion schedules (abstract). Proc Am Soc Clin Oncol 11: 329
Tonato M (1993). Gemcitabine safety overview. In: Gemcitabine novel combination of efficacy and tolerability. Proceedings European Conference Clinical Oncology 7 Satellite Symposium, Jerusalem, Israel. pp 14–16
Mertens WC, Eisenhauer EA, Moore M, et al (1993) Gemcitabine in advanced renal cell carcinoma. A phase II study of the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol 4:331–332
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
De Mulder, P.H.M., Weissbach, L., Jakse, G. et al. Gemcitabine: A phase II study in patients with advanced renal cancer. Cancer Chemother Pharmacol 37, 491–495 (1996). https://doi.org/10.1007/s002800050417
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s002800050417