Abstract
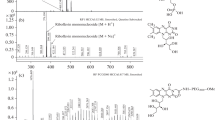
Purpose: Previously we have shown that doxorubicin (Adriamycin, ADR) can be inactivated by light-excited riboflavin. The inactivation of the drug results from its direct oxidation by the excited triplet riboflavin in a type I photosensitization reaction, and 3-methoxysalicylic acid is an ADR breakdown product. In the present study, we investigated the enhancement of this process by histidine and some other imidazole analogs. Methods: ADR solutions containing various concentrations of riboflavin and other agents were exposed to 365 nm light for various time periods and then the absorbance spectrum of ADR was measured by a double beam spectrophotometer. These measurement were used to calculate the half-time of the ADR degradation process. The degraded ADR solutions were analyzed by HPLC. Results: The rate of bleaching of ADR by light-excited riboflavin was enhanced in the presence of histidine in a concentration-dependent manner. This enhancement was more pronounced at higher riboflavin concentrations. Histidine also enhanced the riboflavin-mediated photobleaching of N,N-dimethyl-4-nitrosoaniline (RNO), a compound known to be resistant to oxidation by singlet oxygen but sensitive to oxidation by the trans-annular peroxide of histidine. RNO was found to block the histidine enhancement of the riboflavin-mediated photobleaching of ADR in a competitive manner. Among the imidazole analogs of histidine tested, urocanic acid was found to be the most efficient enhancer of the riboflavin-mediated photobleaching of ADR. Superoxide anion radicals which retard the oxidation of ADR were quenched by urocanic acid but not by histidine. It was shown that the oxidation of ADR by the trans-annular peroxide of histidine resulted in the formation of 3-methoxysalicylic acid. Conclusions: In contrast to singlet oxygen, the trans-annular peroxide, formed by the interaction of histidine and the singlet oxygen produced by photoexcited riboflavin, is an efficient oxidizer of ADR. The enhancement of the riboflavin-mediated photobleaching of ADR by histidine analogs depends on the rate of their conversion to a trans-annular peroxide and on the efficiency of these products in oxidizing ADR. However, for some analogs of histidine, as shown for urocanic acid, other mechanisms could also be involved. The presence of urocanic acid in the skin suggests that significant degradation of ADR could occur in the presence of biologically relevant concentrations of riboflavin if patients treated with ADR are exposed to sunlight. The finding that histidine also enhanced the degradation of ADR to 3-methoxysalicylic acid, suggests that the process of ADR oxidation by the trans-annular peroxides is similar to the direct oxidation of ADR by excited triplet riboflavin.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Ramu, A., Mehta, M., Leaseburg, T. et al. The enhancement of riboflavin-mediated photo-oxidation of doxorubicin by histidine and urocanic acid. Cancer Chemother Pharmacol 47, 338–346 (2001). https://doi.org/10.1007/s002800000236
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s002800000236