Abstract
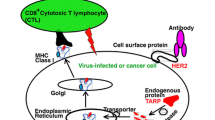
Genetic changes leading to protooncogene activation qualitatively and/or quantitatively alter their gene products and are exclusively or largely restricted to transforming cells and their precursors. The overexpression of HER2 is among those changes and is often detected in adenocarcinomas such as breast, ovarian, lung, and gastric cancer. This provides a rationale for exploring the possibility that HER2 is a target of host immune responses against cancer cells. We have recently demonstrated that HER2 can be a target for tumor-rejecting immune responses against syngeneic murine HER2+ tumor cells. We defined two different peptides, HER2p63–71 and HER2p780–788, with a Kd anchor motif that can induce CD8+ cytotoxic T lymphocytes (CTLs). The growth of HER2+ syngeneic tumors was suppressed in mice immunized with HER2p63–71 or p780–788. Since murine Kd and human HLA-A24 share a similar anchor motif for peptides, HER2p63–71 and HER2p780–788 were examined for induction of CTLs in HLA-A24+ individuals. CD8+ CTL clones specific for these peptides were established and they lysed HER2+ tumor cells in a human leukocyte antigen (HLA)-A24-restricted manner. To elicit specific CD8+ T cell immune responses against cancer, the development of efficient devices to deliver tumor antigen peptides to the major histocompatibility complex (MHC) class I pathway constitutes a central issue. We have developed a novel formula of hydrophobized polysaccharide nanoparticles which can deliver a HER2 oncoprotein containing an epitope peptide to the MHC class I pathway. We designed a simple protein delivery system: cholesteryl group-bearing polysaccharides, mannan or pullulan (CHM or CHP, respectively), complexed with the truncated HER2 protein containing the 147 N-terminal amino acids. These complexes were able to induce CD8+ CTLs against HER2+ tumors. CTLs were MHC class I restricted and specifically recognized HER2p63–71, a part of a truncated HER2 protein used as an immunogen. The complete rejection of tumors also occurred when CHM-HER2 was applied early after tumor implantation. In the effector phase of in vivo tumor rejection, CD8+ T cells played a major role. The results suggest that this unique hydrophobized polysaccharide may help soluble proteins to induce cellular immunity. Such a novel vaccine may be of potential benefit in cancer prevention and cancer therapy.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shiku, H., Wang, L., Ikuta, Y. et al. Development of a cancer vaccine: peptides, proteins, and DNA. Cancer Chemother Pharmacol 46 (Suppl 1), S77–S82 (2000). https://doi.org/10.1007/s002800000179
Issue Date:
DOI: https://doi.org/10.1007/s002800000179