Abstract
Purpose
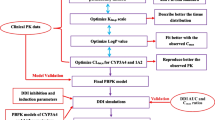
Develop a physiologically based pharmacokinetic (PBPK) model of ivosidenib using in vitro and clinical PK data from healthy participants (HPs), refine it with clinical data on ivosidenib co-administered with itraconazole, and develop a model for patients with acute myeloid leukemia (AML) and apply it to predict ivosidenib drug–drug interactions (DDI).
Methods
An HP PBPK model was developed in Simcyp Population-Based Simulator (version 15.1), with the CYP3A4 component refined based on a clinical DDI study. A separate model accounting for the reduced apparent oral clearance in patients with AML was used to assess the DDI potential of ivosidenib as the victim of CYP3A perpetrators.
Results
For a single 250 mg ivosidenib dose, the HP model predicted geometric mean ratios of 2.14 (plasma area under concentration–time curve, to infinity [AUC0-∞]) and 1.04 (maximum plasma concentration [Cmax]) with the strong CYP3A4 inhibitor, itraconazole, within 1.26-fold of the observed values (2.69 and 1.0, respectively). The AML model reasonably predicted the observed ivosidenib concentration–time profiles across all dose levels in patients. Predicted ivosidenib geometric mean steady-state AUC0-∞ and Cmax ratios were 3.23 and 2.26 with ketoconazole, and 1.90 and 1.52 with fluconazole, respectively. Co-administration of the strong CYP3A4 inducer, rifampin, predicted a greater DDI effect on a single dose of ivosidenib than on multiple doses (AUC ratios 0.35 and 0.67, Cmax ratios 0.91 and 0.81, respectively).
Conclusion
Potentially clinically relevant DDI effects with CYP3A4 inducers and moderate and strong inhibitors co-administered with ivosidenib were predicted. Considering the challenges of conducting clinical DDI studies in patients, this PBPK approach is valuable in ivosidenib DDI risk assessment and management.




Similar content being viewed by others
Data availability
Investigators interested in data sharing and collaboration should contact the corresponding author.
References
Popovici-Muller J, Lemieux RM, Artin E, Saunders JO, Salituro FG, Travins J, Cianchetta G, Cai Z, Zhou D, Cui D, Chen P, Straley K, Tobin E, Wang F, David MD, Penard-Lacronique V, Quivoron C, Saada V, de Botton S, Gross S, Dang L, Yang H, Utley L, Chen Y, Kim H, Jin S, Gu Z, Yao G, Luo Z, Lv X, Fang C, Yan L, Olaharski A, Silverman L, Biller S, Su SM, Yen K (2018) Discovery of AG-120 (ivosidenib): a first-in-class mutant IDH1 inhibitor for the treatment of IDH1 mutant cancers. ACS Med Chem Lett 9(4):300–305
DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, Swords R, Collins RH, Mannis GN, Pollyea DA, Donnellan W, Fathi AT, Pigneux A, Erba HP, Prince GT, Stein AS, Uy GL, Foran JM, Traer E, Stuart RK, Arellano ML, Slack JL, Sekeres MA, Willekens C, Choe S, Wang H, Zhang V, Yen KE, Kapsalis SM, Yang H, Dai D, Fan B, Goldwasser M, Liu H, Agresta S, Wu B, Attar EC, Tallman MS, Stone RM, Kantarjian HM (2018) Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med 378(25):2386–2398
Roboz GJ, DiNardo CD, Stein EM, de Botton S, Mims AS, Prince GT, Altman JK, Arellano ML, Donnellan W, Erba HP, Mannis GN, Pollyea DA, Stein AS, Uy GL, Watts JM, Fathi AT, Kantarjian HM, Tallman MS, Choe S, Dai D, Fan B, Wang H, Zhang V, Yen KE, Kapsalis SM, Hickman D, Liu H, Agresta SV, Wu B, Attar EC, Stone RM (2020) Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood 135(7):463–471
Fan B, Mellinghoff IK, Wen PY, Lowery MA, Goyal L, Tap WD, Pandya SS, Manyak E, Jiang L, Liu G, Nimkar T, Gliser C, Prahl Judge M, Agresta S, Yang H, Dai D (2020) Clinical pharmacokinetics and pharmacodynamics of ivosidenib, an oral, targeted inhibitor of mutant IDH1, in patients with advanced solid tumors. Invest New Drugs 38(2):433–444
Prakash C, Fan B, Altaf S, Agresta S, Liu H, Yang H (2019) Pharmacokinetics, absorption, metabolism, and excretion of [(14)C]ivosidenib (AG-120) in healthy male subjects. Cancer Chemother Pharmacol 83(5):837–848
Dai D, Yang H, Nabhan S, Liu H, Hickman D, Liu G, Zacher J, Vutikullird A, Prakash C, Agresta S, Bowden C, Fan B (2019) Effect of itraconazole, food, and ethnic origin on the pharmacokinetics of ivosidenib in healthy subjects. Eur J Clin Pharmacol 75(8):1099–1108
Jiang X, Wada R, Poland B, Kleijn HJ, Fan B, Liu G, Liu H, Kapsalis S, Yang H, Le K (2020) Population pharmacokinetic and exposure-response analyses of ivosidenib (AG-120) in patients with IDH1-mutant advanced hematologic malignancies. Clin Pharmacol Ther (In review)
Almond LM, Mukadam S, Gardner I, Okialda K, Wong S, Hatley O, Tay S, Rowland-Yeo K, Jamei M, Rostami-Hodjegan A, Kenny JR (2016) Prediction of drug-drug interactions arising from CYP3A induction using a physiologically based dynamic model. Drug Metab Dispos 44(6):821–832
Howgate EM, Rowland Yeo K, Proctor NJ, Tucker GT, Rostami-Hodjegan A (2006) Prediction of in vivo drug clearance from in vitro data. I: impact of inter-individual variability. Xenobiotica 36(6):473–497
Inoue S, Howgate EM, Rowland-Yeo K, Shimada T, Yamazaki H, Tucker GT, Rostami-Hodjegan A (2006) Prediction of in vivo drug clearance from in vitro data. II: potential inter-ethnic differences. Xenobiotica 36(6):499–513
Leil TA, Kasichayanula S, Boulton DW, LaCreta F (2014) Evaluation of 4beta-hydroxycholesterol as a clinical biomarker of CYP3A4 drug interactions using a Bayesian mechanism-based pharmacometric model. CPT Pharmacometrics Syst Pharmacol 3(6):e120
Wagner C, Pan Y, Hsu V, Sinha V, Zhao P (2016) Predicting the effect of CYP3A inducers on the pharmacokinetics of substrate drugs using physiologically based pharmacokinetic (PBPK) modeling: an analysis of PBPK submissions to the US FDA. Clin Pharmacokinet 55(4):475–483
Ke A, Barter Z, Rowland-Yeo K, Almond L (2016) Towards a best practice approach in PBPK modeling: case example of developing a unified efavirenz model accounting for induction of CYPs 3A4 and 2B6. CPT Pharmacometrics Syst Pharmacol 5(7):367–376
Jalava KM, Partanen J, Neuvonen PJ (1997) Itraconazole decreases renal clearance of digoxin. Ther Drug Monit 19(6):609–613
Gorski JC, Vannaprasaht S, Hamman MA, Ambrosius WT, Bruce MA, Haehner-Daniels B, Hall SD (2003) The effect of age, sex, and rifampin administration on intestinal and hepatic cytochrome P450 3A activity. Clin Pharmacol Ther 74(3):275–287
Jones HM, Chen Y, Gibson C, Heimbach T, Parrott N, Peters SA, Snoeys J, Upreti VV, Zheng M, Hall SD (2015) Physiologically based pharmacokinetic modeling in drug discovery and development: a pharmaceutical industry perspective. Clin Pharmacol Ther 97(3):247–262
Luzon E, Blake K, Cole S, Nordmark A, Versantvoort C, Berglund EG (2017) Physiologically based pharmacokinetic modeling in regulatory decision-making at the European Medicines Agency. Clin Pharmacol Ther 102(1):98–105
Sager JE, Yu J, Ragueneau-Majlessi I, Isoherranen N (2015) Physiologically based pharmacokinetic (PBPK) modeling and simulation approaches: a systematic review of published models, applications, and model verification. Drug Metab Dispos 43(11):1823–1837
European Medicines Agency (2012) Guideline on the investigation of drug interactions. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-drug-interactions_en.pdf. Accessed 17 April 2020
U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) (2017) Clinical drug interaction studies — study design, data analysis, and clinical implications guidance for industry. https://www.fda.gov/files/drugs/published/Clinical-Drug-Interaction-Studies-%E2%80%94-Study-Design--Data-Analysis--and-Clinical-Implications-Guidance-for-Industry.pdf. Accessed 17 April 2020
Grimstein M, Yang Y, Zhang X, Grillo J, Huang SM, Zineh I, Wang Y (2019) Physiologically based pharmacokinetic modeling in regulatory science: an update from the U.S. Food and Drug Administration's Office of Clinical Pharmacology. J Pharm Sci 108(1):21–25
Coutant DE, Kulanthaivel P, Turner PK, Bell RL, Baldwin J, Wijayawardana SR, Pitou C, Hall SD (2015) Understanding disease-drug interactions in cancer patients: implications for dosing within the therapeutic window. Clin Pharmacol Ther 98(1):76–86
Cousin L, Berre ML, Launay-Vacher V, Izzedine H, Deray G (2003) Dosing guidelines for fluconazole in patients with renal failure. Nephrol Dial Transplant 18(11):2227–2231
Acknowledgements
We would like to dedicate this article to the memory of our colleague, David Dai. We would like to thank the healthy participants and patients taking part in these studies. Assistance with manuscript preparation was provided by Christine Ingleby, PhD, CMPP, Excel Medical Affairs, Horsham, UK, and supported by Agios.
Funding
This study was supported financially by Agios Pharmaceuticals, Inc.
Author information
Authors and Affiliations
Contributions
All authors performed data analysis and interpretation, as well as manuscript writing, review, and approval.
Corresponding author
Ethics declarations
Conflict of interest
C.P., B.F., K.L., and H.Y. were employees of and stockholders in Agios Pharmaceuticals, Inc., at the time of the study. A.K. is an employee of a contract research organization, Certara UK Ltd.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the studies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bin Fan, Kha Le, and Hua Yang: affiliation at time of study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Prakash, C., Fan, B., Ke, A. et al. Physiologically based pharmacokinetic modeling and simulation to predict drug–drug interactions of ivosidenib with CYP3A perpetrators in patients with acute myeloid leukemia. Cancer Chemother Pharmacol 86, 619–632 (2020). https://doi.org/10.1007/s00280-020-04148-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-020-04148-3