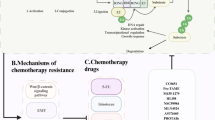
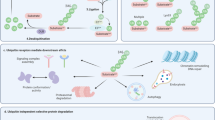
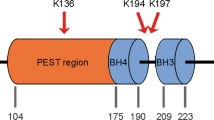
Abstract
Drug resistance is a well-known phenomenon leading to a reduction in the effectiveness of pharmaceutical treatments. Resistance to chemotherapeutic agents can involve various intrinsic cellular processes including drug efflux, increased resistance to apoptosis, increased DNA damage repair capabilities in response to platinum salts or other DNA-damaging drugs, drug inactivation, drug target alteration, epithelial–mesenchymal transition (EMT), inherent cell heterogeneity, epigenetic effects, or any combination of these mechanisms. Deubiquitinating enzymes (DUBs) reverse ubiquitination of target proteins, maintaining a balance between ubiquitination and deubiquitination of proteins to maintain cell homeostasis. Increasing evidence supports an association of altered DUB activity with development of several cancers. Thus, DUBs are promising candidates for targeted drug development. In this review, we outline the involvement of DUBs, particularly ubiquitin-specific proteases, and their roles in drug resistance in different types of cancer. We also review potential small molecule DUB inhibitors that can be used as drugs for cancer treatment.


Similar content being viewed by others
Abbreviations
- USP:
-
Ubiquitin-specific protease
- UPP:
-
Ubiquitin proteosomal pathway
- DUBs:
-
Deubiquitinases
- UB:
-
Ubiquitination
- UBD:
-
Ubiquitin-binding domain
- ZnF-UBP domain:
-
Zinc finger ubiquitin-specific protease domain
- UIM:
-
Ubiquitin-interacting motif
- UBA domain:
-
Ubiquitin-associated domain
- UCHs:
-
Ubiquitin C-terminal hydrolases
- OTUs:
-
Ovarian tumor proteases
- MJD:
-
Machado–Joseph domain protease
- FDA:
-
Food and drug administration
- PI:
-
Proteasome inhibitor
- MM:
-
Multiple myeloma
- NSCLC:
-
Non-small cell lung cancer
- Bcl-xL:
-
B-cell lymphoma-extra large
- Bcl-2:
-
B-cell lymphoma 2
- CDDP:
-
Cis-diaminedichloroplatin (II)
- USP:
-
Ubiquitin-specific peptidase
- UAF1:
-
WD repeat-containing protein 48
- LCLs:
-
Lymphoblastoid cell lines
- PTEN:
-
Phosphatase and tensin homolog
- PARP:
-
Poly ADP ribose polymerase
- MDM2:
-
Murine double minute oncogene
- ER:
-
Estrogen receptor
- EGFR:
-
Epidermal growth factor receptor
- RTK:
-
Receptor tyrosine kinase
- BAG3:
-
BAG family molecular chaperone regulator 3
- Mcl-1:
-
Myeloid leukemia cell differentiation protein
- TAM:
-
Tamoxifen
- ERα:
-
Estrogen receptor α
- FLT3-ITD:
-
FMS-like tyrosine kinase 3 internal tandem duplication
- USP9x:
-
Ubiquitin-specific peptidase 9x
- siRNA:
-
Small interfering RNA
- TKD:
-
Tyrosine kinase domain
- CAM-DR:
-
Cell adhesion-mediated drug resistance
- WM:
-
Waldenstrom macroglobulinemia
- BCR:
-
B-cell receptor
- MYD88:
-
Myeloid differentiation primary response 88
- PBMC:
-
Peripheral blood mononuclear cell
- MDR:
-
Multidrug resistance
- HCC:
-
Hepatocellular carcinoma
- CSCs:
-
Cancer stem cells
- SIRT1:
-
Silent mating type information regulation 2 homolog 1
- MRP1:
-
Multidrug resistance-associated protein 1
- NAFLD:
-
Non-alcoholic fatty liver disease
- TAK1:
-
Transforming growth factor beta-activated kinase 1
- TLS:
-
Translesion synthesis repair pathway
References
Yewdell JW, Schubert U, Antón LC et al (2000) Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature 404:770–774
Hershko A (2005) The ubiquitin system for protein degradation and some of its roles in the control of the cell division cycle. Cell Death Differ 12:1191–1197
Coux O, Tanaka K, Goldberg AL (1996) Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem 65:801–847
Hershko A, Ciechanover A, Varshavsky A (2000) The ubiquitin system. Nat Med 6:1073–1081
Ciechanover A, Orian A, Schwartz AL (2000) Ubiquitin-mediated proteolysis: biological regulation via destruction. BioEssays 22:442–451
Ciechanover A (2005) Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol 6:79–86
Zhang L, Xu B, Qiang Y et al (2015) Overexpression of deubiquitinating enzyme USP28 promoted non-small cell lung cancer growth. J Cell Mol Med 19:799–805
Cox JL, Wilder PJ, Wuebben EL et al (2014) Context-dependent function of the deubiquitinating enzyme USP9X in pancreatic ductal adenocarcinoma. Cancer Biol Ther 15:1042–1052
Komander D, Clague MJ, Urbé S (2009) Breaking the chains: structure and function of the deubiquitinases. Nat Rev Mol Cell Biol 10:550–563
Pfoh R, Lacdao IK, Saridakis V (2015) Deubiquitinases and the new therapeutic opportunities offered to cancer. Endocr Relat Cancer 22:T35–54
Wilkinson KD (2009) DUBs at a glance. J Cell Sci 122:2325–2329
Ramakrishna S, Suresh B, Baek KH (2011) The role of deubiquitinating enzymes in apoptosis. Cell Mol Life Sci 68:15–26
Farshi P, Deshmukh RR, Nwankwo JO et al (2015) Deubiquitinases (DUBs) and DUB inhibitors: a patent review. Expert Opin Ther Pat 25(10):1191–1208
Xie X (2019) Deubiquitinase as potential targets for cancer immunotherapy. J Cell Immunol 1:1–3
Yuan T, Yan F, Ying M et al (2018) Inhibition of ubiquitin-specific proteases as a novel anticancer therapeutic strategy. Front Pharmacol 9:1–10
D’Arcy P, Wang X, Linder S (2015) Deubiquitinase inhibition as a cancer therapeutic strategy. Pharmacol Ther 147:32–54
Adams J (2004) The proteasome: a suitable antineoplastic target. Nat Rev Cancer 4:349–360
Song MS, Salmena L, Carracedo A et al (2008) The deubiquitinylation and localization of PTEN are regulated by a HAUSP-PML network. Nature 455:813–817
Nijman SMB, Luna-Vargas MPA, Velds A et al (2005) A genomic and functional inventory of deubiquitinating enzymes. Cell 123:773–786
Zhou MJ, Chen FZ, Chen HC (2014) Ubiquitination involved enzymes and cancer. Med Oncol 31:93
Voutsadakis IA (2012) Ubiquitination and the ubiquitin-proteasome system as regulators of transcription and transcription factors in epithelial mesenchymal transition of cancer. Tumour Biol 33:897–910
Lander GC, Estrin E, Matyskiela ME et al (2012) Complete subunit architecture of the proteasome regulatory particle. Nature 482:186–191
Haq S, Suresh B, Ramakrishna S (2018) Deubiquitylating enzymes as cancer stem cell therapeutics. Biochim Biophys Acta Rev Cancer 1869:1–10
Hoeller D, Hecker CM, Dikic I (2006) Ubiquitin and ubiquitin-like proteins in cancer pathogenesis. Nat Rev Cancer 6:776–788
Nicholson B, Marblestone JG, Butt TR, Mattern MR (2007) Deubiquitinating enzymes as novel anticancer targets. Futur Oncol 3:191–199
Nicholson B, Leach CA, Goldenberg SJ et al (2008) Characterization of ubiquitin and ubiquitin-like-protein isopeptidase activities. Protein Sci 17:1035–1043
Kapuria V, Peterson LF, Fang D et al (2010) Deubiquitinase inhibition by small-molecule WP1130 triggers aggresome formation and tumor cell apoptosis. Cancer Res 70:9265–9276
McClurg UL, Robson CN (2015) Deubiquitinating enzymes as oncotargets. Oncotarget 6:9657–9668
Hicke L, Schubert HL, Hill CP (2005) Ubiquitin-binding domains. Nat Rev Mol Cell Biol 6:610–621
Fraile JM, Quesada V, Rodríguez D et al (2012) Deubiquitinases in cancer: new functions and therapeutic options. Oncogene 31:2373–2388
Brnjic S, Mazurkiewicz M, Fryknäs M et al (2014) Induction of tumor cell apoptosis by a proteasome deubiquitinase inhibitor is associated with oxidative stress. Antioxid Redox Signal 21:2271–2285
Sgorbissa A, Potu H, Brancolini C (2010) Isopeptidases in anticancer therapy: looking for inhibitors. Am J Transl Res 2:235–247
Goldberg AL (2007) Functions of the proteasome: from protein degradation and immune surveillance to cancer therapy. Biochem Soc Trans 35:12–17
Kisselev AF, Goldberg AL (2001) Proteasome inhibitors: from research tools to drug candidates. Chem Biol 8:739–758
Guédat P, Colland F (2007) Patented small molecule inhibitors in the ubiquitin proteasome system. BMC Biochem 8(Suppl):S14
Li T, Yan B, Ma Y et al (2018) Ubiquitin-specific protease 4 promotes hepatocellular carcinoma progression via cyclophilin A stabilization and deubiquitination. Cell Death Dis 9:148
Pal A, Donato NJ (2014) Ubiquitin-specific proteases as therapeutic targets for the treatment of breast cancer. Breast Cancer Res 16:5–11
Young MJ, Hsu KC, Lin TE et al (2019) The role of ubiquitin-specific peptidases in cancer progression. J Biomed Sci 26:1–14
Melo-Cardenas J, Zhang Y, Zhang DD, Fang D (2016) Ubiquitin-specific peptidase 22 functions and its involvement in disease. Oncotarget 7:44848–44856
Weisberg EL, Schauer NJ, Yang J et al (2017) Inhibition of USP10 induces degradation of oncogenic FLT3. Nat Chem Biol 13:1207–1215
Tyagi N, Tyagi M, Pachauri M, Ghosh PC (2015) Potential therapeutic applications of plant toxin-ricin in cancer: challenges and advances. Tumor Biol 36:8239–8246
Srivastava SK, Bhardwaj A, Arora S et al (2015) MicroRNA-345 induces apoptosis in pancreatic cancer cells through potentiation of caspase-dependent and -independent pathways. Br J Cancer 113:660–668
Arora S, Tyagi N, Bhardwaj A et al (2015) Silver nanoparticles protect human keratinocytes against UVB radiation-induced DNA damage and apoptosis: potential for prevention of skin carcinogenesis. Nanomedicine 11:1265–1275
Dou Q, Zonder J (2014) Overview of proteasome inhibitor-based anti-cancer therapies: perspective on bortezomib and second generation proteasome inhibitors versus future generation inhibitors of ubiquitin-proteasome system. Curr Cancer Drug Targets 14:517–536
Chauhan D, Catley L, Li G et al (2005) A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from Bortezomib. Cancer Cell 8:407–419
Crosas B (2014) Deubiquitinating enzyme inhibitors and their potential in cancer therapy. Curr Cancer Drug Targets 14:506–516
Lim K-H, Baek K-H (2013) Deubiquitinating enzymes as therapeutic targets in cancer. Curr Pharm Des 19:4039–4052
Kale AJ, Moore BS (2012) Molecular mechanisms of acquired proteasome inhibitor resistance. J Med Chem 55:10317–10327
Niewerth D, Jansen G, Assaraf YG et al (2015) Molecular basis of resistance to proteasome inhibitors in hematological malignancies. Drug Resist Updat 18:18–35
Neslund-dudas C, Mitra B, Dou QP (2013) From bortezomib to other inhibitors of the proteasome and beyond. Curr Pharm Des 19:4025–4038
Hagenbuchner J, Ausserlechner MJ, Porto V et al (2010) The anti-apoptotic protein BCL2L1/Bcl-xL is neutralized by pro-apoptotic PMAIP1/noxa in neuroblastoma, thereby determining bortezomib sensitivity independent of prosurvival MCL1 expression. J Biol Chem 285:6904–6912
Chhabra S (2017) Novel proteasome inhibitors and histone deacetylase inhibitors: progress in myeloma therapeutics. Pharmaceuticals 10:40
Hungria VTM, Crusoe EQ, Bittencourt RI et al (2019) New proteasome inhibitors in the treatment of multiple myeloma. Hematol Transfus Cell Ther 41:76–83
Manasanch EE, Orlowski RZ (2017) Proteasome inhibitors in cancer therapy. Nat Rev Clin Oncol 14:417–433
Naymagon L, Abdul-Hay M (2016) Novel agents in the treatment of multiple myeloma: a review about the future. J Hematol Oncol 9:52
Allegra A, Alonci A, Gerace D et al (2014) New orally active proteasome inhibitors in multiple myeloma. Leuk Res 38:1–9
Moreau P, de Wit E (2017) Recent progress in relapsed multiple myeloma therapy: implications for treatment decisions. Br J Haematol 179:198–218
Szalat R, Munshi NC (2019) Novel agents in multiple myeloma. Cancer J 25:45–53
Kubiczkova L, Pour L, Sedlarikova L et al (2014) Proteasome inhibitors—molecular basis and current perspectives in multiple myeloma. J Cell Mol Med 18:947–961
Sourisseau T, Helissey C, Lefebvre C et al (2016) Translational regulation of the mRNA encoding the ubiquitin peptidase USP1 involved in the DNA damage response as a determinant of Cisplatin resistance. Cell Cycle 15:295–302
Reyes-Turcu FE, Ventii KH, Wilkinson KD (2009) Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu Rev Biochem 78:363–397
Morra F, Merolla F, Criscuolo D et al (2019) CCDC6 and USP7 expression levels suggest novel treatment options in high-grade urothelial bladder cancer. J Exp Clin Cancer Res 38:1–14
Malapelle U, Morra F, Ilardi G et al (2017) USP7 inhibitors, downregulating CCDC6, sensitize lung neuroendocrine cancer cells to PARP-inhibitor drugs. Lung Cancer 107:41–49
Morra F, Luise C, Merolla F et al (2015) FBXW7 and USP7 regulate CCDC6 turnover during the cell cycle and affect cancer drugs susceptibility in NSCLC. Oncotarget 6:12697–12709
Nicholson B, Suresh Kumar KG (2011) The multifaceted roles of USP7: new therapeutic opportunities. Cell Biochem Biophys 60:61–68
Chauhan D, Tian Z, Nicholson B et al (2012) A small molecule inhibitor of ubiquitin-specific protease-7 induces apoptosis in multiple myeloma cells and overcomes bortezomib resistance. Cancer Cell 22:345–358
Cummins JM, Rago C, Kohli M et al (2004) Tumour suppression: disruption of HAUSP gene stabilizes p53. Nature 416:648–653
Fang S, Jensen JP, Ludwig RL et al (2000) Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J Biol Chem 275:8945–8951
Itahana K, Mao H, Jin A et al (2007) Targeted inactivation of Mdm2 RING finger E3 ubiquitin ligase activity in the mouse reveals mechanistic insights into p53 regulation. Cancer Cell 12:355–366
Kon N, Kobayashi Y, Li M et al (2010) Inactivation of HAUSP in vivo modulates p53 function. Oncogene 29:1270–1279
Meulmeester E, Maurice MM, Boutell C et al (2005) Loss of HAUSP-mediated deubiquitination contributes to DNA damage-induced destabilization of Hdmx and Hdm2. Mol Cell 18:565–576
Anderson KC (2007) Targeted therapy of multiple myeloma based upon tumor-microenvironmental interactions. Exp Hematol 35:155–162
Tian X, Isamiddinova NS, Peroutka RJ et al (2011) Characterization of selective ubiquitin and ubiquitin-like protease inhibitors using a fluorescence-based multiplex assay format. Assay Drug Dev Technol 9:165–173
Turnbull AP, Ioannidis S, Krajewski WW et al (2017) Molecular basis of USP7 inhibition by selective small molecule inhibitors. Nature 550:481–486
Wang Z, Kang W, You Y et al (2019) USP7: novel drug target in cancer therapy. Front Pharmacol 10:1–15
Kategaya L, Di Lello P, Rougé L et al (2017) USP7 small-molecule inhibitors interfere with ubiquitin binding. Nature 550:534–538
Byun S, Lee SY, Lee J et al (2013) USP8 is a novel target for overcoming gefitinib resistance in lung cancer. Clin Cancer Res 19:3894–3904
Bleyer A, Welch HG (2012) Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med 367:1998–2005
Vesuna F, Lisok A, Kimble B et al (2012) Twist contributes to hormone resistance in breast cancer by downregulating estrogen receptor-α. Oncogene 31:3223–3234
Schwarz LJ, Fox EM, Balko JM et al (2014) LYN-activating mutations mediate antiestrogen resistance in estrogen receptor-positive breast cancer. J Clin Invest 124:5490–5502
Santos-Martínez N, Díaz L, Ordaz-Rosado D et al (2014) Calcitriol restores antiestrogen responsiveness in estrogen receptor negative breast cancer cells: A potential new therapeutic approach. BMC Cancer 14:230
Fishman J, Osborne MP, Telang NT (1995) The role of estrogen in mammary carcinogenesis. Ann N Y Acad Sci 768:91–100
Massarweh S, Osborne CK, Creighton CJ et al (2008) Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res 68:826–833
Oosterkamp HM, Hijmans EM, Brummelkamp TR et al (2014) USP9X downregulation renders breast cancer cells resistant to tamoxifen. Cancer Res 74:3810–3820
Fu P, Du F, Liu Y et al (2017) WP1130 increases cisplatin sensitivity through inhibition of usp9x in estrogen receptor-negative breast cancer cells. Am J Transl Res 9:1783–1791
Tian Z, D’Arcy P, Wang X et al (2014) A novel small molecule inhibitor of deubiquitylating enzyme USP14 and UCHL5 induces apoptosis in multiple myeloma and overcomes bortezomib resistance. Blood 123:706–716
Lee BH, Lee MJ, Park S et al (2010) Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature 467:179–184
Reeder SB, Hu HH, Sirlin CB et al (1999) Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood 93:1658–1667
Furukawa Y, Kikuchi J (2015) Molecular pathogenesis of multiple myeloma. Int J Clin Oncol 20:413–422
Raninga PV, Di Trapani G, Vuckovic S et al (2015) Inhibition of thioredoxin 1 leads to apoptosis in drug-resistant multiple myeloma. Oncotarget 6:15410–15424
Tsubaki M, Takeda T, Ogawa N et al (2015) Overexpression of survivin via activation of ERK1/2, Akt, and NF-κB plays a central role in vincristine resistance in multiple myeloma cells. Leuk Res 39:445–452
Hazlehurst LA, Valkov N, Wisner L et al (2001) Reduction in drug-induced DNA double-strand breaks associated with β1 integrin-mediated adhesion correlates with drug resistance in U937 cells. Blood 98:1897–1903
Taylor ST, Hickman JA, Dive C (2000) Epigenetic determinants of resistance to etoposide regulation of Bcl- x(L) and Bax by tumor microenvironmental factors. J Natl Cancer Inst 92:18–23
Hazlehurst LA, Argilagos RF, Emmons M et al (2006) Cell adhesion to fibronectin (CAM-DR) influences acquired mitoxantrone resistance in U937 cells. Cancer Res 66:2338–2345
Shinji S, Naito Z, Ishiwata S et al (2006) Ubiquitin-specific protease 14 expression in colorectal cancer is associated with liver and lymph node metastases. Oncol Rep 15:539–543
Chuensumran U, Saelee P, Punyarit P et al (2011) Ubiquitin-specific protease 14 expression associated with intrahepatic cholangiocarcinoma cell differentiation. Asian Pac J Cancer Prev 12:775–779
Yang Y, Hou JQ, Qu LY et al (2007) Differential expression of USP2, USP14 and UBE4A between ovarian serous cystadenocarcinoma and adjacent normal tissues. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 23:504–506
Wu N, Liu C, Bai C et al (2013) Over-expression of deubiquitinating enzyme USP14 in lung adenocarcinoma promotes proliferation through the accumulation of β-catenin. Int J Mol Sci 14:10749–10760
Treon SP, Tripsas CK, Meid K et al (2015) Ibrutinib in previously treated Waldenström’s macroglobulinemia. N Engl J Med 372:1430–1440
Paulus A, Akhtar S, Caulfield TR et al (2016) Coinhibition of the deubiquitinating enzymes, USP14 and UCHL5, with VLX1570 is lethal to ibrutinib- or bortezomib-resistant Waldenstrom macroglobulinemia tumor cells. Blood Cancer J 6:e492
Aftab O, Engskog MK, Haglof J et al (2014) NMR spectroscopy-based metabolic profiling of drug-induced changes in vitro can discriminate between pharmacological classes. J Chem Inf Model 54:3251–3258
Schmidt M, Altdörfer V, Schnitte S et al (2019) The deubiquitinase inhibitor b-AP15 and its effect on phenotype and function of monocyte-derived dendritic cells. Neoplasia 21:653–664
Selvaraju K, Mazurkiewicz M, Wang X et al (2015) Inhibition of proteasome deubiquitinase activity: a strategy to overcome resistance to conventional proteasome inhibitors? Drug Resist Updat 21–22:20–29
Coughlin K, Anchoori R, Iizuka Y et al (2014) Small-molecule RA-9 inhibits proteasome-associated DUBs and ovarian cancer in vitro and in vivo via exacerbating unfolded protein responses. Clin Cancer Res 20:3174–3186
Ling S, Li J, Shan Q et al (2017) USP22 mediates the multidrug resistance of hepatocellular carcinoma via the SIRT1/AKT/MRP1 signaling pathway. Mol Oncol 11:682–695
Chen HC, Jeng YM, Yuan RH et al (2012) SIRT1 promotes tumorigenesis and resistance to chemotherapy in hepatocellular carcinoma and its expression predicts poor prognosis. Ann Surg Oncol 19:2011–2019
Harris SL, Levine AJ (2005) The p53 pathway: positive and negative feedback loops. Oncogene 24:2899–2908
Zou Q, Jin J, Hu H et al (2014) USP15 stabilizes MDM2 to mediate cancer-cell survival and inhibit antitumor T cell responses. Nat Immunol 15:562–570
An S, Zhao LP, Shen LJ et al (2017) USP18 protects against hepatic steatosis and insulin resistance through its deubiquitinating activity. Hepatology 66:1866–1884
Davis MI, Pragani R, Fox JT et al (2016) Small molecule inhibition of the ubiquitin-specific protease USP2 accelerates cyclin D1 degradation and leads to cell cycle arrest in colorectal cancer and mantle cell lymphoma models. J Biol Chem 291:24628–24640
Xu X, Huang A, Cui X et al (2019) Ubiquitin specific peptidase 5 regulates colorectal cancer cell growth by stabilizing Tu translation elongation factor. Theranostics 9:4208–4220
Zhi Y, Shoujun H, Yuanzhou S et al (2012) STAT3 repressed USP7 expression is crucial for colon cancer development. FEBS Lett 586:3013–3017
Khan OM, Carvalho J, Spencer-Dene B et al (2018) The deubiquitinase USP9X regulates FBW7 stability and suppresses colorectal cancer. J Clin Invest 128:1326–1337
Li W, Cui K, Prochownik EV, Li Y (2018) The deubiquitinase USP21 stabilizes MEK2 to promote tumor growth. Cell Death Dis 9:482
Kosinsky RL, Zerche M, Saul D et al (2019) USP22 exerts tumor-suppressive functions in colorectalS cancer by decreasing mTOR activity. Cell Death Differ. https://doi.org/10.1038/s41418-019-0420-8
Burkhart RA, Peng Y, Norris ZA et al (2013) Mitoxantrone targets human ubiquitin-specific peptidase 11 (USP11) and is a potent inhibitor of pancreatic cancer cell survival. Mol Cancer Res 11:901–911
Cai J, Liu T, Huang P et al (2017) USP39, a direct target of microRNA-133a, promotes progression of pancreatic cancer via the AKT pathway. Biochem Biophys Res Commun 486:184–190
Hou K, Zhu Z, Wang Y et al (2016) Overexpression and biological function of ubiquitin-spSecific protease 42 in gastric cancer. PLoS ONE 11:1–11
Nishimura S, Oki E, Ando K et al (2017) High ubiquitin-specific protease 44 expression induces DNA aneuploidy and provides independent prognostic information in gastric cancer. Cancer Med 6:1453–1464
Gu YY, Yang M, Zhao M et al (2015) The de-ubiquitinase UCHL1 promotes gastric cancer metastasis via the Akt and Erk1/2 pathways. Tumor Biol 36:8379–8387
Zhong M, Jiang Q, Jin R (2018) USP4 expression independently predicts favorable survival in lung adenocarcinoma. IUBMB Life 70:670–677
Ma X, Qi W, Pan H et al (2018) Overexpression of USP5 contributes to tumorigenesis in non-small cell lung cancer via the stabilization of β-catenin protein. Am J Cancer Res 8:2284–2295
Cui S, Fan L, Li Y, et al (2019) Targeting the USP1 dependent KDM4A protein stability as a potential prostate cancer therapy. (In Press). https://doi.org/10.1111/cas.14375
Guo F, Zhang C, Wang F et al (2019) Deubiquitinating enzyme USP33 restrains docetaxel-induced apoptosis via stabilising the phosphatase DUSP1 in prostate cancer. Cell Death Differ. https://doi.org/10.1038/s41418-019-0473-8
Lee JK, Chang N, Yoon Y et al (2016) USP1 targeting impedes GBM growth by inhibiting stem cell maintenance and radioresistance. Neuro Oncol 18:37–47
Pal A, Young MA, Donato NJ (2014) Emerging potential of therapeutic targeting of ubiquitin-specific proteases in the treatment of cancer. Cancer Res 74:4955–4966
Ding K, Ji J, Zhang X et al (2019) RNA splicing factor USP39 promotes glioma progression by inducing TAZ mRNA maturation. Oncogene 38:6414–6428
Qiu GZ, Mao XY, Ma Y et al (2018) Ubiquitin-specific protease 22 acts as an oncoprotein to maintain glioma malignancy through deubiquitinating B cell-specific Moloney murine leukemia virus integration site 1 for stabilization. Cancer Sci 109:2199
Oikonomaki M, Bady P, Hegi ME (2017) Ubiquitin Specific Peptidase 15 (USP15) suppresses glioblastoma cell growth via stabilization of HECTD1 E3 ligase attenuating WNT pathway activity. Oncotarget 8:110490–110502
Hwang SJ, Lee HW, Kim HR et al (2016) Ubiquitin-specific protease 4 controls metastatic potential through β-catenin stabilization in brain metastatic lung adenocarcinoma. Sci Rep 6:1–13
Hussain S, Zhang Y, Galardy PJ (2009) DUBs and cancer: the role of deubiquitinating enzymes as oncogenes, non-oncogenes and tumor suppressors. Cell Cycle 8:1688–1697
Guo J, Shinriki S, Su Y et al (2014) Hypoxia suppresses cylindromatosis (CYLD) expression to promote inflammation in glioblastoma: possible link to acquired resistance to anti-VEGF therapy. Oncotarget 5:6353–6364
Wang X, Zhang Q, Wang Y et al (2018) Clinical significance of ubiquitin specific protease 7 (USP7) in predicting prognosis of hepatocellular carcinoma and its functional mechanisms. Med Sci Monit 24:1742–1750
Zhang Y, vanDeursen J, Galardy PJ (2011) Overexpression of ubiquitin specific protease 44 (USP44) induces chromosomal instability and is frequently observed in human T-Cell leukemia. PLoS ONE 6(8):e23389
Yasunaga J, Lin FC, Lu X, Jeang K-T (2011) Ubiquitin-specific peptidase 20 targets TRAF6 and human T cell leukemia virus type 1 tax to negatively regulate NF-B signaling. J Virol 85:6212–6219
Liu Y, Xu X, Lin P et al (2019) Inhibition of the deubiquitinase USP9x induces pre-B cell homeobox 1 (PBX1) degradation and thereby stimulates prostate cancer cell apoptosis. J Biol Chem 294:4572–4582
Shan H, Li X, Xiao X et al (2018) USP7 deubiquitinates and stabilizes NOTCH1 in T-cell acute lymphoblastic leukemia. Signal Transduct Target Ther 3:1–10
Chae YC, Jung H, Kim JY et al (2019) Ubiquitin-specific peptidase 3 induces TPA-mediated leukemia cell differentiation via regulating H2AK119ub. Anim Cells Syst 23:311–317
Mistry H, Hsieh G, Buhrlage SJ et al (2013) Small-molecule inhibitors of USP1 target ID1 degradation in leukemic cells. Mol Cancer Ther 12:2651–2662
Hahn M, Bürckert JP, Luttenberger CA et al (2018) Aberrant splicing of the tumor suppressor CYLD promotes the development of chronic lymphocytic leukemia via sustained NF-κB signaling. Leukemia 32:72–82
Nijman SMB, Huang TT, Dirac AMG et al (2005) The deubiquitinating enzyme USP1 regulates the fanconi anemia pathway. Mol Cell 17:331–339
Sonego M, Pellarin I, Costa A et al (2019) USP1 links platinum resistance to cancer cell dissemination by regulating snail stability. Sci Adv 5:eaav3235
Kim SY, Kwon SK, Lee SY, Baek KH (2018) Ubiquitin-specific peptidase 5 and ovarian tumor deubiquitinase 6A are differentially expressed in p53+/+ and p53−/− HCT116 cells. Int J Oncol 52:1705–1714
Ma M, Yu N (2016) Ubiquitin-specific protease 7 expression is a prognostic factor in epithelial ovarian cancer and correlates with lymph node metastasis. Onco Targets Ther 9:1559–1569
Yan C, Yuan J, Xu J et al (2019) Ubiquitin-specific peptidase 39 regulates the process of proliferation and migration of human ovarian cancer via p53/p21 pathway and EMT. Med Oncol 36:1–13
Jin C, Yu W, Lou X et al (2013) UCHL1 is a putative tumor suppressor in ovarian cancer cells and contributes to cisplatin resistance. J Cancer 4:662–670
Jacq X, Kemp M, Martin NMB, Jackson SP (2013) Deubiquitylating enzymes and DNA damage response pathways. Cell Biochem Biophys 67:25–43
Fang CL, Lin CC, Chen HK et al (2018) Ubiquitin-specific protease 3 overexpression promotes gastric carcinogenesis and is predictive of poor patient prognosis. Cancer Sci 109:3438–3449
Wu Y, Qin Q, Li F et al (2019) USP3 promotes breast cancer cell proliferation by deubiquitinating KLF5. J Biol Chem 294:17837–17847
Il YS, Kim HH, Yoon JH et al (2015) Ubiquitin specific protease 4 positively regulates the WNT/β-catenin signaling in colorectal cancer. Mol Oncol 9:1834–1851
Nakajima S, Lan L, Wei L et al (2014) Ubiquitin-specific protease 5 is required for the efficient repair of DNA double-strand breaks. PLoS ONE 9(1):e84899
Madana B, Walkerb MP, Young R et al (2016) USP6 oncogene promotes Wnt signaling by deubiquitylating Frizzleds. Proc Natl Acad Sci USA 113:E2945–E2954
Cheng J, Yang H, Fang J et al (2015) Molecular mechanism for USP7-mediated DNMT1 stabilization by acetylation. Nat Commun 6:1–11
Berlin I, Higginbotham KM, Dise RS et al (2010) The deubiquitinating enzyme USP8 promotes trafficking and degradation of the chemokine receptor 4 at the sorting endosome. J Biol Chem 285:37895–37908
Zhang E, Shen B, Mu X et al (2016) Ubiquitin-specific protease 11 (USP11) functions as a tumor suppressor through deubiquitinating and stabilizing VGLL4 protein. Am J Cancer Res 6:2901–2909
Xu X, Liu J, Shen C et al (2017) The role of ubiquitin-specific protease 14 (USP14) in cell adhesion-mediated drug resistance (CAM-DR) of multiple myeloma cells. Eur J Haematol 98:4–12
Wang C, Yang C, Ji J et al (2017) Deubiquitinating enzyme USP20 is a positive regulator of claspin and suppresses the malignant characteristics of gastric cancer cells. Int J Oncol 50:1136–1146
Haq S, Das S, Kim DH et al (2019) The stability and oncogenic function of LIN28A are regulated by USP28. Biochim Biophys Acta Mol Basis Dis 1865:599–610
Van Leuken RJ, Luna-Vargas MP, Sixma TK et al (2008) Usp39 is essential for mitotic spindle checkpoint integrity and controls mRNA-levels of aurora B. Cell Cycle 7:2710–2719
Cetkovská K, Šustová H, Uldrijan S (2017) Ubiquitin-specific peptidase 48 regulates Mdm2 protein levels independent of its deubiquitinase activity. Sci Rep 7:1–9
Zhou Z, Yao X, Pang S et al (2018) The deubiquitinase UCHL5/UCH37 positively regulates Hedgehog signaling by deubiquitinating Smoothened. J Mol Cell Biol 10:243–257
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Education (2017M3A9C6061361, 2018M3A9H3022412, 2017R1A2B2008727 and 2017M3A9B3061830).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no potential conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tanguturi, P., Kim, KS. & Ramakrishna, S. The role of deubiquitinating enzymes in cancer drug resistance. Cancer Chemother Pharmacol 85, 627–639 (2020). https://doi.org/10.1007/s00280-020-04046-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-020-04046-8