Abstract
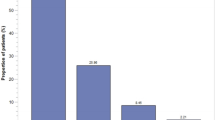
The risk of potential drug–drug interactions (PDI) is poorly studied in oncology. We included 105 patients with advanced non-small-cell lung cancer (NSCLC), 100 patients with advanced breast cancer (BC) and 100 patients of the palliative care unit (PCU) receiving systemic palliative treatment between 2010 and 2015. All patients suffered from advanced incurable cancer and received basic palliative care. PDI were assessed using the hospINDEX of all drugs approved in Switzerland in combination with a specific drug interaction software. Primary study objective was to assess the prognostic impact of PDI per patient cohort using Kaplan–Meier statistics. The median number of comedications was 5 (range 0–15). Major-risk PDI were detected in 74 patients (24.3%). The number of comedications was significantly associated with PDI (p < 0.0001). Major-risk PDI increased from 14% in patients with < 4 comedications to 24% in patients with 4–7 comedications, 40% with 8–11 comedications and 67% in patients with > 11 comedications. Median overall survival (OS) was 8.6 months in NSCLC, 33 months in BC and 1.2 months in PCU patients. PDI were significantly associated with inferior OS in BC (HR = 1.32, 95% CI 1.01–1.74, p = 0.049), but not in NSCLC (HR = 1.11, 95% CI 0.84–1.47, p = 0.45) or PCU (HR = 1.12, 95% CI 0.86–1.45, p = 0.41). PDI remained significantly associated with OS in BC (HR = 1.32, p = 0.049) in the adjusted model. In conclusion, PDI are frequent in patients with advanced cancer and increased caution with polypharmacy is warranted when treating such patients.




Similar content being viewed by others
References
Beijnen JH, Schellens JH (2004) Drug interactions in oncology. Lancet Oncol 5(8):489–496. https://doi.org/10.1016/S1470-2045(04)01528-1
Scripture CD, Figg WD (2006) Drug interactions in cancer therapy. Nat Rev Cancer 6(7):546–558. https://doi.org/10.1038/nrc1887
McLeod HL (1997) Therapeutic drug monitoring opportunities in cancer therapy. Pharmacol Ther 74(1):39–54
Ratz Bravo AE, Tchambaz L, Krahenbuhl-Melcher A, Hess L, Schlienger RG, Krahenbuhl S (2005) Prevalence of potentially severe drug–drug interactions in ambulatory patients with dyslipidaemia receiving HMG-CoA reductase inhibitor therapy. Drug Saf 28(3):263–275
Guthrie B, Makubate B, Hernandez-Santiago V, Dreischulte T (2015) The rising tide of polypharmacy and drug–drug interactions: population database analysis 1995–2010. BMC Med 13:74. https://doi.org/10.1186/s12916-015-0322-7
Leucuta SE, Vlase L (2006) Pharmacokinetics and metabolic drug interactions. Curr Clin Pharmacol 1(1):5–20
Yoshida K, Maeda K, Sugiyama Y (2013) Hepatic and intestinal drug transporters: prediction of pharmacokinetic effects caused by drug–drug interactions and genetic polymorphisms. Annu Rev Pharmacol Toxicol 53:581–612. https://doi.org/10.1146/annurev-pharmtox-011112-140309
Zhang H, Davis CD, Sinz MW, Rodrigues AD (2007) Cytochrome P450 reaction-phenotyping: an industrial perspective. Expert Opin Drug Metab Toxicol 3(5):667–687. https://doi.org/10.1517/17425255.3.5.667
Ramos-Esquivel A, Viquez-Jaikel A, Fernandez C (2017) Potential drug–drug and herb–drug interactions in patients with cancer: a prospective study of medication surveillance. J Oncol Pract. https://doi.org/10.1200/JOP.2017.020859
Segal EM, Flood MR, Mancini RS, Whiteman RT, Friedt GA, Kramer AR, Hofstetter MA (2014) Oral chemotherapy food and drug interactions: a comprehensive review of the literature. J Oncol Pract 10(4):e255–e268. https://doi.org/10.1200/JOP.2013.001183
Kang SP, Ratain MJ (2010) Inconsistent labeling of food effect for oral agents across therapeutic areas: differences between oncology and non-oncology products. Clin Cancer Res 16(17):4446–4451. https://doi.org/10.1158/1078-0432.CCR-10-0663
Szmulewitz RZ, Ratain MJ (2013) Playing Russian roulette with tyrosine kinase inhibitors. Clin Pharmacol Ther 93(3):242–244. https://doi.org/10.1038/clpt.2012.245
Riechelmann RP, Del Giglio A (2009) Drug interactions in oncology: how common are they? Ann Oncol 20(12):1907–1912. https://doi.org/10.1093/annonc/mdp369
Peters S, Zimmermann S, Adjei AA (2014) Oral epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small cell lung cancer: comparative pharmacokinetics and drug–drug interactions. Cancer Treatm Rev 40(8):917–926. https://doi.org/10.1016/j.ctrv.2014.06.010
van Leeuwen RW, van Gelder T, Mathijssen RH, Jansman FG (2014) Drug–drug interactions with tyrosine-kinase inhibitors: a clinical perspective. Lancet Oncol 15(8):e315–e326. https://doi.org/10.1016/S1470-2045(13)70579-5
Riechelmann RP, Zimmermann C, Chin SN, Wang L, O’Carroll A, Zarinehbaf S, Krzyzanowska MK (2008) Potential drug interactions in cancer patients receiving supportive care exclusively. J Pain Symptom Manag 35(5):535–543. https://doi.org/10.1016/j.jpainsymman.2007.06.009
Ussai S, Petelin R, Giordano A, Malinconico M, Cirillo D, Pentimalli F (2015) A pilot study on the impact of known drug–drug interactions in cancer patients. J Exp Clin Cancer Res 34:89. https://doi.org/10.1186/s13046-015-0201-2
Yeoh TT, Tay XY, Si P, Chew L (2015) Drug-related problems in elderly patients with cancer receiving outpatient chemotherapy. J Geriatr Oncol 6(4):280–287. https://doi.org/10.1016/j.jgo.2015.05.001
Ussai S, Sparta MC (2014) Adverse drug events: how information technology will meet the challenges of pharmacovigilance. Value Health 17(7):A750. https://doi.org/10.1016/j.jval.2014.08.193
McCune JS, Hatfield AJ, Blackburn AA, Leith PO, Livingston RB, Ellis GK (2004) Potential of chemotherapy–herb interactions in adult cancer patients. Support Care Cancer 12(6):454–462. https://doi.org/10.1007/s00520-004-0598-1
Popa MA, Wallace KJ, Brunello A, Extermann M, Balducci L (2014) Potential drug interactions and chemotoxicity in older patients with cancer receiving chemotherapy. J Geriatr Oncol 5(3):307–314. https://doi.org/10.1016/j.jgo.2014.04.002
Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel JM, Sundararajan V (2011) Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 173(6):676–682. https://doi.org/10.1093/aje/kwq433
Karlen S, Eschmann E, Blaser J (2014) Entscheidungsunterstützung beim Medikamentenprozess: Wie umfangreich sind die hospINDEX-Stammdaten? Swiss Med Inf 30
van Leeuwen RW, Swart EL, Boven E, Boom FA, Schuitenmaker MG, Hugtenburg JG (2011) Potential drug interactions in cancer therapy: a prevalence study using an advanced screening method. Ann Oncol 22(10):2334–2341. https://doi.org/10.1093/annonc/mdq761
Voll ML, Yap KD, Terpstra WE, Crul M (2010) Potential drug–drug interactions between anti-cancer agents and community pharmacy dispensed drugs. Pharm World Sci 32(5):575–580. https://doi.org/10.1007/s11096-010-9410-0
Bulsink A, Imholz AL, Brouwers JR, Jansman FG (2013) Characteristics of potential drug-related problems among oncology patients. Int J Clin Pharm 35(3):401–407. https://doi.org/10.1007/s11096-012-9747-7
Kruse V, Somers A, Van Bortel L, De Both A, Van Belle S, Rottey S (2014) Sunitinib for metastatic renal cell cancer patients: observational study highlighting the risk of important drug–drug interactions. J Clin Pharm Ther 39(3):259–265. https://doi.org/10.1111/jcpt.12134
Fernandez de Palencia Espinosa MA, Diaz Carrasco MS, Fuster Soler JL, Ruiz Merino G, De la Rubia Nieto MA, Espuny Miro A (2014) Pharmacoepidemiological study of drug–drug interactions in onco-hematological pediatric patients. Int J Clin Pharm 36(6):1160–1169. https://doi.org/10.1007/s11096-014-0011-1
Stoll P, Kopittke L (2015) Potential drug–drug interactions in hospitalized patients undergoing systemic chemotherapy: a prospective cohort study. Int J Clin Pharm 37(3):475–484. https://doi.org/10.1007/s11096-015-0083-6
Rompelman FM, Smit AA, Franssen EJ, Crul M (2016) Drug–drug interactions of cytostatics with regular medicines in lung cancer patients. J Oncol Pharm Pract. https://doi.org/10.1177/1078155216664200
Alsanad SM, Howard RL, Williamson EM (2016) An assessment of the impact of herb–drug combinations used by cancer patients. BMC Complement Altern Med 16(1):393. https://doi.org/10.1186/s12906-016-1372-x
Keller KL, Franquiz MJ, Duffy AP, Trovato JA (2016) Drug–drug interactions in patients receiving tyrosine kinase inhibitors. J Oncol Pharm Pract. https://doi.org/10.1177/1078155216682311
Balk TE, van der Sijs IH, van Gelder T, Janssen JJB, van der Sluis IM, van Leeuwen RWF, Engels FK (2017) Drug–drug interactions in pediatric oncology patients. Pediatr Blood Cancer. https://doi.org/10.1002/pbc.26410
Meek IL, Vonkeman HE, Kasemier J, Movig KL, van de Laar MA (2013) Interference of NSAIDs with the thrombocyte inhibitory effect of aspirin: a placebo-controlled, ex vivo, serial placebo-controlled serial crossover study. Eur J Clin Pharmacol 69(3):365–371. https://doi.org/10.1007/s00228-012-1370-y
Martinez-Moreno R, Aguilar M, Wendl C, Bazner H, Ganslandt O, Henkes H (2016) Fatal thrombosis of a flow diverter due to ibuprofen-related antagonization of acetylsalicylic acid. Clin Neuroradiol 26(3):355–358. https://doi.org/10.1007/s00062-015-0487-7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests concerning this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hoemme, A., Barth, H., Haschke, M. et al. Prognostic impact of polypharmacy and drug interactions in patients with advanced cancer. Cancer Chemother Pharmacol 83, 763–774 (2019). https://doi.org/10.1007/s00280-019-03783-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03783-9