Abstract
Purpose
This phase I trial evaluated the safety, pharmacokinetic profile, and antitumor activity of investigational oral TORC1/2 inhibitor TAK-228 plus paclitaxel, with/without trastuzumab, in patients with advanced solid malignancies.
Methods
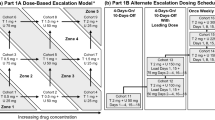
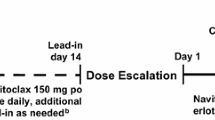
Sixty-seven patients received TAK-228 6–40 mg via three dosing schedules; once daily for 3 days (QDx3d QW) or 5 days per week (QDx5d QW), and once weekly (QW) plus paclitaxel 80 mg/m2 (dose-escalation phase, n = 47) and with/without trastuzumab 2 mg/kg (expansion phase, n = 20). Doses were escalated using a modified 3 + 3 design, based upon dose-limiting toxicities in cycle 1.
Results
TAK-228 pharmacokinetics exhibited dose-dependent increase in exposure when dosed with paclitaxel and no apparent differences when administered with or 24 h after paclitaxel. Dose-limiting toxicities were dehydration, diarrhea, stomatitis, fatigue, rash, thrombocytopenia, neutropenia, leukopenia, and nausea. The maximum tolerated dose of TAK-228 was determined as 10-mg QDx3d QW; the expansion phase proceeded with 8-mg QDx3d QW. Overall, the most common grade ≥3 drug-related toxicities were neutropenia (21%), diarrhea (12%), and hyperglycemia (12%). Of 54 response-evaluable patients, eight achieved partial response and six had stable disease lasting ≥6 months.
Conclusion
TAK-228 demonstrated a safety profile consistent with other TORC inhibitors and promising preliminary antitumor activity in a range of tumor types; no meaningful difference was noted in the pharmacokinetics of TAK-228 when administered with or 24 h after paclitaxel. These findings support further investigation of TAK-228 in combination with other agents including paclitaxel, with/without trastuzumab, in patients with advanced solid tumors.
Clinicaltrials.gov identifier
NCT01351350.

Similar content being viewed by others
References
Dazert E, Hall MN (2011) mTOR signaling in disease. Curr Opin Cell Biol 23:744–755
Hsieh AC, Liu Y, Edlind MP et al (2012) The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature 485:55–61
Wu R, Hu TC, Rehemtulla A et al (2011) Preclinical testing of PI3K/AKT/mTOR signaling inhibitors in a mouse model of ovarian endometrioid adenocarcinoma. Clin Cancer Res 17:7359–7372
Zoncu R, Efeyan A, Sabatini DM (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12:21–35
Cantrell DA (2001) Phosphoinositide 3-kinase signalling pathways. J Cell Sci 114:1439–1445
Fresno Vara JA, Casado E, de Castro J et al (2004) PI3K/Akt signalling pathway and cancer. Cancer Treat Rev 30:193–204
Huang J, Manning BD (2009) A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans 37:217–222
Pene F, Claessens YE, Muller O et al (2002) Role of the phosphatidylinositol 3-kinase/Akt and mTOR/P70S6-kinase pathways in the proliferation and apoptosis in multiple myeloma. Oncogene 21:6587–6597
Sabatini DM (2006) mTOR and cancer: insights into a complex relationship. Nat Rev Cancer 6:729–734
Liang J, Slingerland JM (2003) Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle 2:339–345
Benjamin D, Colombi M, Moroni C et al (2011) Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov 10:868–880
Vu C, Fruman DA (2010) Target of rapamycin signaling in leukemia and lymphoma. Clin Cancer Res 16:5374–5380
Robb VA, Karbowniczek M, Klein-Szanto AJ et al (2007) Activation of the mTOR signaling pathway in renal clear cell carcinoma. J Urol 177(1):346–352
Dal CJ, Zancai P, Terrin L et al (2008) Distinct functional significance of Akt and mTOR constitutive activation in mantle cell lymphoma. Blood 111(10):5142–5151
Xiao L, Wang YC, Li WS et al (2009) The role of mTOR and phospho-p70S6K in pathogenesis and progression of gastric carcinomas: an immunohistochemical study on tissue microarray. J Exp Clin Cancer Res 28:152–158
Matsubara S, Ding Q, Miyazaki Y et al (2013) mTOR plays critical roles in pancreatic cancer stem cells through specific and stemness-related functions. Sci Rep 3:3230
Mahadevan D, Chiorean EG, Harris WB et al (2012) Phase I pharmacokinetic and pharmacodynamic study of the pan-PI3K/mTORC vascular targeted pro-drug SF1126 in patients with advanced solid tumours and B-cell malignancies. Eur J Cancer 48(18):3319–3327
Sarker D, Ang JE, Baird R et al (2015) First-in-human phase I study of pictilisib (GDC-0941), a potent pan-class I phosphatidylinositol-3-kinase (PI3K) inhibitor, in patients with advanced solid tumors. Clin Cancer Res 21:77–86
Yang Q, Modi P, Newcomb T et al (2015) Idelalisib: first-in-class PI3K delta inhibitor for the treatment of chronic lymphocytic leukemia, small lymphocytic leukemia, and follicular lymphoma. Clin Cancer Res 21:1537–1542
Agarwal R, Koenig K, Rohren E et al (2014) Combined antiangiogenic and mammalian target of rapamycin inhibitor targeted therapy in metaplastic breast cancer harboring a PIK3CA mutation. J Breast Cancer 17:287–290
Bellmunt J, Puente J, de Garcia MJ et al (2014) SEOM clinical guidelines for the treatment of renal cell carcinoma. Clin Transl Oncol 16:1043–1050
Signorovitch J, Swallow E, Kantor E et al (2013) Everolimus and sunitinib for advanced pancreatic neuroendocrine tumors: a matching-adjusted indirect comparison. Exp Hematol Oncol 2:32
Thoreen CC, Kang SA, Chang JW et al (2009) An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem 284:8023–8032
Wan X, Harkavy B, Shen N et al (2007) Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene 26(13):1932–1940
Carracedo A, Ma L, Teruya-Feldstein J et al (2008) Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest 118(9):3065–3074
Figlin RA, Kaufmann I, Brechbiel J (2013) Targeting PI3K and mTORC2 in metastatic renal cell carcinoma: new strategies for overcoming resistance to VEGFR and mTORC1 inhibitors. Int J Cancer 133(4):788–796
Carracedo A, Pandolfi PP (2008) The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene 27(41):5527–5541
Feldman ME, Apsel B, Uotila A et al (2009) Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol 7:e38
O’Reilly KE, Rojo F, She QB et al (2006) mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 66:1500–1508
Tabernero J, Rojo F, Calvo E et al (2008) Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol 26:1603–1610
Korets SB, Musa F, Curtin J et al (2014) Dual mTORC1/2 inhibition in a preclinical xenograft tumor model of endometrial cancer. Gynecol Oncol 132(2):468–473
Zheng B, Mao JH, Qian L et al (2015) Pre-clinical evaluation of AZD-2014, a novel mTORC1/2 dual inhibitor, against renal cell carcinoma. Cancer Lett 357(2):468–475
Ingels A, Zhao H, Thong AE et al (2014) Preclinical trial of a new dual mTOR inhibitor, MLN0128, using renal cell carcinoma tumorgrafts. Int J Cancer 134:2322–2329
Gokmen-Polar Y, Liu Y, Toroni RA et al (2012) Investigational drug MLN0128, a novel TORC1/2 inhibitor, demonstrates potent oral antitumor activity in human breast cancer xenograft models. Breast Cancer Res Treat 136:673–682
Lou HZ, Weng XC, Pan HM et al (2014) The novel mTORC1/2 dual inhibitor INK-128 suppresses survival and proliferation of primary and transformed human pancreatic cancer cells. Biochem Biophys Res Commun 450:973–978
Zhang H, Dou J, Yu Y et al (2015) mTOR ATP-competitive inhibitor INK128 inhibits neuroblastoma growth via blocking mTORC signaling. Apoptosis 20:50–62
Slotkin EK, Patwardhan PP, Vasudeva SD et al (2015) MLN0128, an ATP-competitive mTOR kinase inhibitor with potent in vitro and in vivo antitumor activity, as potential therapy for bone and soft-tissue sarcoma. Mol Cancer Ther 14:395–406
Kannan K, Fabrey R, Cooper J et al (2013) MLN0128, an investigational mTORC1/2 inhibitor, demonstrates potent antitumor activity alone and in combination with paclitaxel in preclinical models of endometrial cancer. Mol Cancer Ther 12 (abstract B198)
Chiang CT, Yeh PY, Gao M et al (2010) Combinations of mTORC1 inhibitor RAD001 with gemcitabine and paclitaxel for treating non-Hodgkin lymphoma. Cancer Lett 298(2):195–203
Shafer A, Zhou C, Gehrig PA et al (2010) Rapamycin potentiates the effects of paclitaxel in endometrial cancer cells through inhibition of cell proliferation and induction of apoptosis. Int J Cancer 126(5):1144–1154
Miller TW, Forbes JT, Shah C et al (2009) Inhibition of mammalian target of rapamycin is required for optimal antitumor effect of HER2 inhibitors against HER2-overexpressing cancer cells. Clin Cancer Res 15(23):7266–7276
Garcia-Garcia C, Ibrahim YH, Serra V et al (2012) Dual mTORC1/2 and HER2 blockade results in antitumor activity in preclinical models of breast cancer resistant to anti-HER2 therapy. Clin Cancer Res 18:2603–2612
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Ghobrial I et al (2016) MLN0128, an investigational oral dual TORC1/2 inhibitor: a phase I dose escalation study in patients with relapsed or refractory multiple myeloma, non-Hodgkin lymphoma, or Waldenström’s macroglobulinemia. Am J Hematol 91:400–405
Infante J, Tabernero J, Cervantes A et al (2013) A phase 1, dose-escalation study of MLN0128, an investigational oral mammalian target of rapamycin complex 1/2 (mTORC1/2) catalytic inhibitor, in patients (pts) with advanced non-hematologic malignancies. Mol Cancer Ther 12 (abstract C252)
Shapiro GI, Rodon J, Bedell C et al (2014) Phase I safety, pharmacokinetic, and pharmacodynamic study of SAR245408 (XL147), an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. Clin Cancer Res 20(1):233–245
Rodon J, Dienstmann R, Serra V et al (2013) Development of PI3K inhibitors: lessons learned from early clinical trials. Nat Rev Clin Oncol 10(3):143–153
Infante J, Tabernero J, Burris H et al (2012) A phase I, open label, dose-escalation study of an oral mammalian target of rapamycin inhibitor INK128 administered once daily in patients with advanced malignancies. Cancer Res 72 (abstract 5588)
Tabernero J, Cervantes A, Gordon M et al (2012) A phase I, open label, dose escalation study of oral mammalian target of rapamycin inhibitor INK128 administered by intermittent dosing regimens in patients with advanced malignancies. Cancer Res 72 (abstract CT-02)
Xu R, Nakano K, Iwasaki H et al (2011) Dual blockade of phosphatidylinositol 3′-kinase and mitogen-activated protein kinase pathways overcomes paclitaxel-resistance in colorectal cancer. Cancer Lett 306:151–160
Acknowledgements
The authors would like to thank all the study participants and their families, as well as the investigators and site staff involved in the study. The authors also acknowledge Ana Limon of Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, who contributed to the editorial and scientific content of the manuscript. The authors would also like to acknowledge the writing support of Dawn L. Lee of FireKite, an Ashfield company, part of UDG Healthcare plc, in the development of this manuscript, which was funded by Millennium Pharmaceuticals, Inc., and complied with Good Publication Practice 3 guidelines (Battisti et al., Ann Intern Med 2015;163:461–4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
Conflict of interest
HA Burris, S Pant, PB Murphy, and SF Jones disclose no potential conflicts of interest. CD Kurkjian participated in an advisory board meeting for Halozyme Therapeutics Inc. (consultant/advisory role). L Hart discloses research funding to institution from Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. JR Infante discloses participation in a consultant/advisory role for Takeda on behalf of institution. R Neuwirth, CG Patel, and F Zohren are employees of Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. CG Patel and F Zohren also disclose stock ownership (Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited).
Ethical approval and informed consent
The study was approved by the institutional review board at each site and conducted per the Declaration of Helsinki, the International Conference on Harmonisation, and Good Clinical Practice guidelines. All patients provided written informed consent.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Burris, H.A., Kurkjian, C.D., Hart, L. et al. TAK-228 (formerly MLN0128), an investigational dual TORC1/2 inhibitor plus paclitaxel, with/without trastuzumab, in patients with advanced solid malignancies. Cancer Chemother Pharmacol 80, 261–273 (2017). https://doi.org/10.1007/s00280-017-3343-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3343-4