Abstract
Purpose
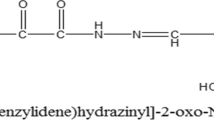
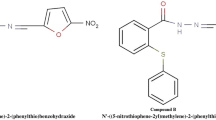
The hydrazide backbone is a well-known structural core system found in a broad range of biologically activated compounds. Among which, the compounds with anticancer activity have been cited in a number of studies. With this object in mind, we focused on the in vitro and in vivo anticancer potential of two novel hydrazide derivatives bearing furan or thiophen substituents (compounds 1 and 2).
Methods
The cytotoxic property was evaluated using MTT assay against MCF-7 human breast adenocarcinoma cell line, while the in vivo antitumor activity was investigated in BALB/c mice bearing 4T1 mammary carcinoma cells. Flow cytometry was used for cell cycle analysis, and detection of apoptosis was examined by Annexin-V-FLUOS/PI assay. Protein expression of Bax, Bcl-2 and procaspase-3 was estimated by Western blotting.
Results
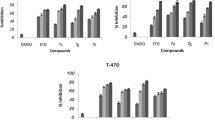
Compounds 1 and 2 were found to be cytotoxic towards breast cancer cells presenting IC50 values of 0.7 and 0.18 µM, respectively, and selectivity over normal fibroblast cells. Our findings further indicated that 2 × IC50 concentrations of both compounds induce early stage apoptosis and increase percentage of sub-G1 population in MCF-7 cells at 48 h. An elevation in Bax/Bcl-2 ratio and caspase-3 cleavage suggested that apoptosis induced by the two compounds is through a caspase- and mitochondrial-dependent pathway. In the in vivo study, compounds 1 and 2 at doses of 10 and 1 mg/Kg/day, respectively, significantly hindered the growth of tumor after 3 weeks of i.p. administration, when compared to vehicle-treated mice.
Conclusion
Collectively, the great potential exhibited by compound 2 could make it a promising chemotherapeutic candidate for human cancers, especially for breast cancer.






Similar content being viewed by others
References
Bray F, Ren JS, Masuyer E, Ferlay J (2013) Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer 132:1133–1145
Forouzanfar MH, Foreman KJ, Delossantos AM, Lozano R, Lopez AD, Murray CJL, Naghavi M (2011) Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. The Lancet 378(9801):1461–1484
Hajrezaie M, Paydar MJ, Looi CY, Moghadamtousi S, Hassandarvish P, Salga MS, Karimian H, Shams K, Zahedifard M, Majid NA (2015) Apoptotic effect of novel Schiff Based CdCl2 (C14H21N3O2) complex is mediated via activation of the mitochondrial pathway in colon cancer cells. Sci Rep 5:90–97
Marsh S, McLeod HL (2007) Pharmacogenetics and oncology treatment for breast cancer. Expert Opin Pharmacother 8:119–127
Kennedy SG, Wanger AJ, Conzen SD, Jordan J, Bellacosa A, Tsichlis PN, Hay N (1997) The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev 11:701–713
Low SW, Lin AW (2000) Apoptosis in cancer. Carcinogenesis 21(3):485–495
Wardakhan WW, EL-Sayed NN, Mohareb RM (2013) Synthesis and anti-tumor evaluation of novel hydrazide-hydrazone derivatives. Acta Pharmaceut 63:45–57
Bharti SK, Nath G, Tilak R, Singh SK (2010) Synthesis, anti-bacterial and anti-fungal activities of some novel Schiff bases containing 2, 4-disubstituted thiazole ring. Eur J Med Chem 45:651–660
Loncle C, Brunel JM, Vidal N, Dherbomez M, Letourneux Y (2004) Synthesis and antifungal activity of cholesterol-hydrazone derivatives. Eur J Med Chem 39:1067–1071
Papakonstantinou-Garoufalias S, Pouli N, Marakos P, Chytyroglou-ladas A (2002) Synthesis antimicrobial and antifungal activity of some new 3-substituted derivatives of 4-(2, 4-dichlorophenyl)-5-adamantyl-1H-1, 2, 4-triazole. Il Farmaco 57:973–977
Vicini P, Zani F, Cozzini P, Doytchinova I (2002) Hydrazones of 1, 2-benzisothiazole hydrazides: synthesis, antimicrobial activity and QSAR investigations. Eur J Med Chem 37:553–564
Sridhar SK, Pandeya SN, Stables JP, Ramesh A (2002) Anticonvulsant activity of hydrazones, schiff and mannich bases of isatin derivatives. Eur J Pharm Sci 16:129–132
Almasirad A, Hosseini R, Jalalizadeh H, Rahimi-Moghaddam Z, Abaeian N, Janafrooz M, Abbaspour M, Ziaee V, Dalvandi A, Shafiee A (2006) Synthesis and analgesic activity of 2-phenoxybenzoic acid N-phenylanthranilic acid hydrazones. Biol Pharm Bull 29(6):1180–1185
Kaymakçıoğlu BK, Rollas S (2002) Synthesis, characterization and evaluation of antituberculosis activity of some hydrazones. Il Farmaco 57:595–599
Vicini P, Incerti M, La Colla P, Lodda R (2009) Anti-HIV evaluation of benzo [d] isothiazole hydrazones. Eur J Med Chem 44:1801–1807
Rahman VM, Mukhtar S, Ansari WH, Lemiere G (2005) Synthesis stereochemistry and biological activity of some novel long alkyl chain substituted thiazolidin-4-ones and thiazan-4-one from 10-undecenoic acid hydrazide. Eur J Med Chem 40:173–184
Dimmock JR, Vashishtha SC, Stables JP (2000) Anticonvulsant properties of various acetylhydrazones, oxamoylhydrazones and semicarbazones derived from aromatic and unsaturated carbonyl compounds. Eur J Med Chem 35:241–248
Kaushik D, Khan SA, Chawla G, Kumar S (2010) N′-[(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl) methylene] 2/4-substituted hydrazides: synthesis and anticonvulsant activity. Eur J Med Chem 45:3943–3949
KüçükgüzelSG Mazi A, Sahin F, Ozturk S, Stables J (2003) Synthesis and biological activities of diflunisal hydrazide–hydrazones. Eur J Med Chem 38:1005–1013
Melnyk P, Leroux V, Sergheraerta C, Grellier P (2006) Design, synthesis and in vitro antimalarial activity of an acylhydrazone library. Bioorg Med Chem Lett 16(1):31–35
Menendez C, Chollet A, Rodriguez F, Inard C, Pasca MR, Lherbet C, Baltas M (2012) Chemical synthesis and biological evaluation of triazole derivatives as inhibitors of InhA and antituberculosis agents. Eur J Med Chem 52:275–283
Bingul M, Tan O, Gardner CR, Sutton SK, Arndt GM, Marshall GM, Cheung BB, Kumar N, Black DS (2016) Synthesis, characterization and anti-cancer activity of hydrazide derivatives incorporating a quinoline moiety. Molecules 21(7):916
Cihan-Üstündağ G, Şatana D, Özhan G, Çapan G (2016) Indole-based hydrazide–hydrazones and 4-thiazolidinones: synthesis and evaluation as antitubercular and anticancer agents. J Enzyme Inhib Med Chem 31(3):369–380
Almasirad A, Samiee-Sadr S, Shafiee A (2011) Synthesis and antimycobacterial activity of 2-(Phenylthio) benzoylarylhydrazone derivatives. Iran J Pharm Res 10(4):727–731
Tavakolfar S, Musavi E, Almasirad A, Amanzadeh A, Atyabi SM, Yaghamii P, Samiee-sadr S, Salimi M (2016) In vitro anticancer effects of two new potent hydrazide compounds in leukemic cells. Anti-Cancer Agents Med Chem 16:1646–1651
Devarajan E, Sahin AA, Chen JS, Krishnamurthy RR, Aggarwal N, Brun AM, Sapino A, Zhang F, Sharma D, Yang XH (2002) Down-regulation of caspase 3 in breast cancer: a possible mechanism for chemoresistance. Oncogene 21:8843–8851
Kroemer G, Reed JC (2000) Mitochondrial control of cell death. Nat Med 6:513–519
Breckenridge DG, Xue D (2004) Regulation of mitochondrial membrane permeabilization by BCL-2 family proteins and caspases. Curr Opin Cell Biol 16:647–652
Abdul Aziz MY, Abu N, Yeap SK, Ho WY, Omar AR, Ismail NH, Ahmad S, Pirozyan MR, Akhtar NM, Alitheen NB (2016) Combinatorial cytotoxic effects of damnacanthal and doxorubicin against human breast cancer MCF-7 cells in vitro. Molecules 21(9):E1228
Enari M, Sakahiro H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S (1998) A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391:43–50
Roopashree R, Mohan CD, Swaroop TR, Jagadish S, Raghava B, Balaji KS, Jayarama S, Rangappa KS (2015) Novel synthetic bisbenzimidazole that targets angiogenesis in Ehrlich ascites carcinoma bearing mice. Bioorg Med Chem Lett 25:2589–2593
Bedia KK, Elcin O, Seda U, Fatma K, Nathaly S, Sevin R, Dimoglo A (2006) Synthesis and characterization of novel hydrazide–hydrazones and the study of their structure–antituberculosis activity. Eur J Med Chem 41(11):1253–1261
Matysiak J, Juszczak M, Karpinska MM, Langer E, Walczak K, Lemieszek MK, Skrzypek A, Niewiadomy A, Rzeski W (2015) Synthesis of 2-(2, 4-dihydroxyphenyl) thieno-1, 3-thiazin-4-ones, their lipophilicity and anticancer activity in vitro. Mol Divers 19(4):725–736
Wu S, Liu B, Zhang Q, Liu J, Zhou W, Wang C, Li M, Bao S, Zhu R (2013) Dihydromyricetin reduced Bcl-2 expression via p53 in human hepatoma HepG2 cells. PLoS One 8(11):e76886
Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, Hoffman B, Reed JC (1994) Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene 9:1799–1805
Das Mukherjee D, Kumar NM, Tantak MP, Das A, Ganguli A, Datta S, Kumar D, Chakrabarti G (2016) Development of novel bis (indolyl)-hydrazide–hydrazone derivatives as potent microtubule-targeting cytotoxic agents against A549 lung cancer cells. Biochemistry 55:3020–3035
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was financially supported by Pasteur Institute of Iran.
Conflict of interest
The authors have declared that there is no conflict of interests to disclose.
Ethical approval
All applicable national and institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Mousavi, E., Tavakolfar, S., Almasirad, A. et al. In vitro and in vivo assessments of two novel hydrazide compounds against breast cancer as well as mammary tumor cells. Cancer Chemother Pharmacol 79, 1195–1203 (2017). https://doi.org/10.1007/s00280-017-3318-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3318-5